4.3 Cancer
Altered Cell Growth
Cells within the human body are exposed to many factors that can significantly impact their growth and function. Two categories of altered cellular growth are benign and malignant tumors, each possessing distinct characteristics and implications. See Figure 4.10[1] for an illustration comparing benign and malignant tumors.
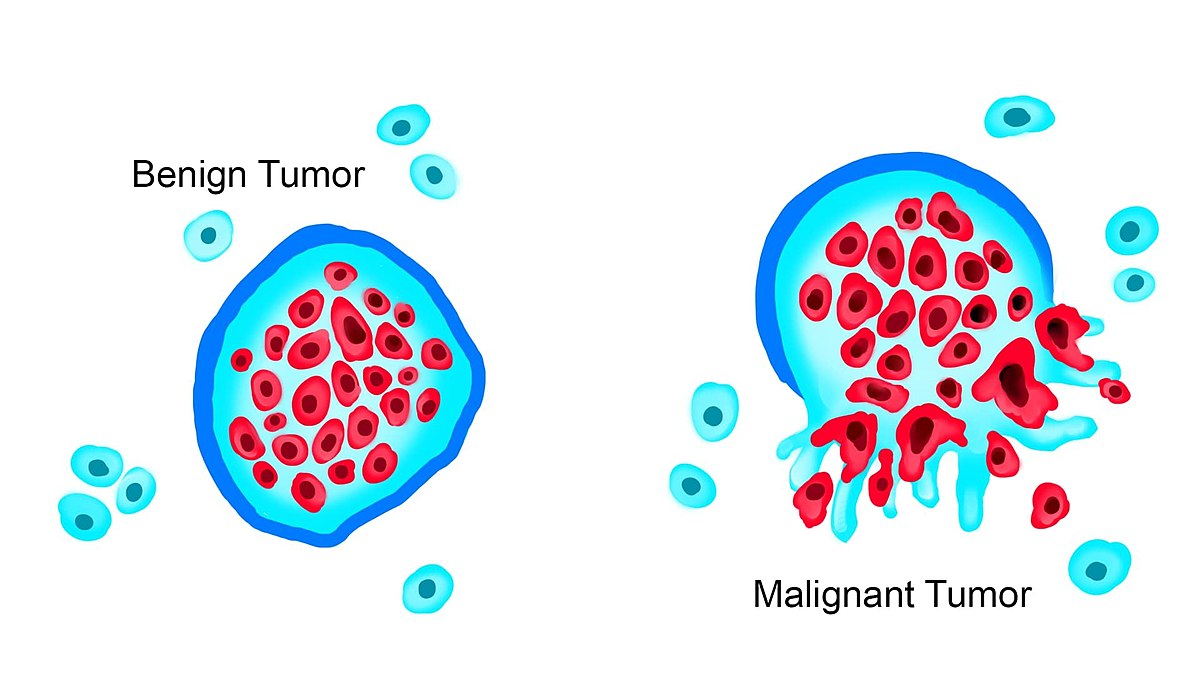
When cellular growth and function deviate from their normal patterns, cells are categorized as abnormal. The term benign refers to abnormal growths that grow and divide in a controlled manner and do not spread outside their location because they are encapsulated, as demonstrated in Figure 4.10.[2] Examples of benign tumors include moles, uterine fibroid tumors, skin tags, endometriosis, and nasal polyps. Benign growths usually involve normal cells growing in locations or at times that are irregular but cause little harm to surrounding tissues.
Malignant cells refer to cancerous developments within the body.[3] Malignancies can be solid tumors or abnormal growth patterns in the blood or lymphatic fluids. These cancer cells not only exhibit abnormal growth patterns but can also invade and impact other body tissues, called metastasis. This type of abnormal cellular growth is considered serious and can lead to death if left untreated.[4]
Malignant cells are the most dangerous due to their ability to metastasize and impact other organs and tissues. When cells metastasize, they typically spread through the blood or lymphatic system. They can also spread during medical procedures due to cross-contamination of surgical instruments or techniques, referred to as iatrogenic metastasis. The spread of the cancer cells and the potential rapid replication and invasion in other areas of the body complicate medical treatment, making it more difficult to rid the body of these abnormal invasive cells.
The distinction between benign and malignant growths is reflected in the medical interventions and prognosis. Benign growth is typically managed through close monitoring and/or minimal surgical interventions. For example, a benign tumor may be surgically removed with no further medical treatment required. However, malignant growths require prompt medical interventions like chemotherapy and radiation to prevent tumor progression and metastasis to other areas of the body.
Metastasis
Metastasis of cancer cells can occur in different parts of the body; however, there are certain organs and tissues that are commonly impacted by each type of cancer. See Figure 4.11[5] for an illustration of metastasis of a primary tumor to the brain, lungs, or liver. Most cancer deaths are caused by metastasis to these major organs.
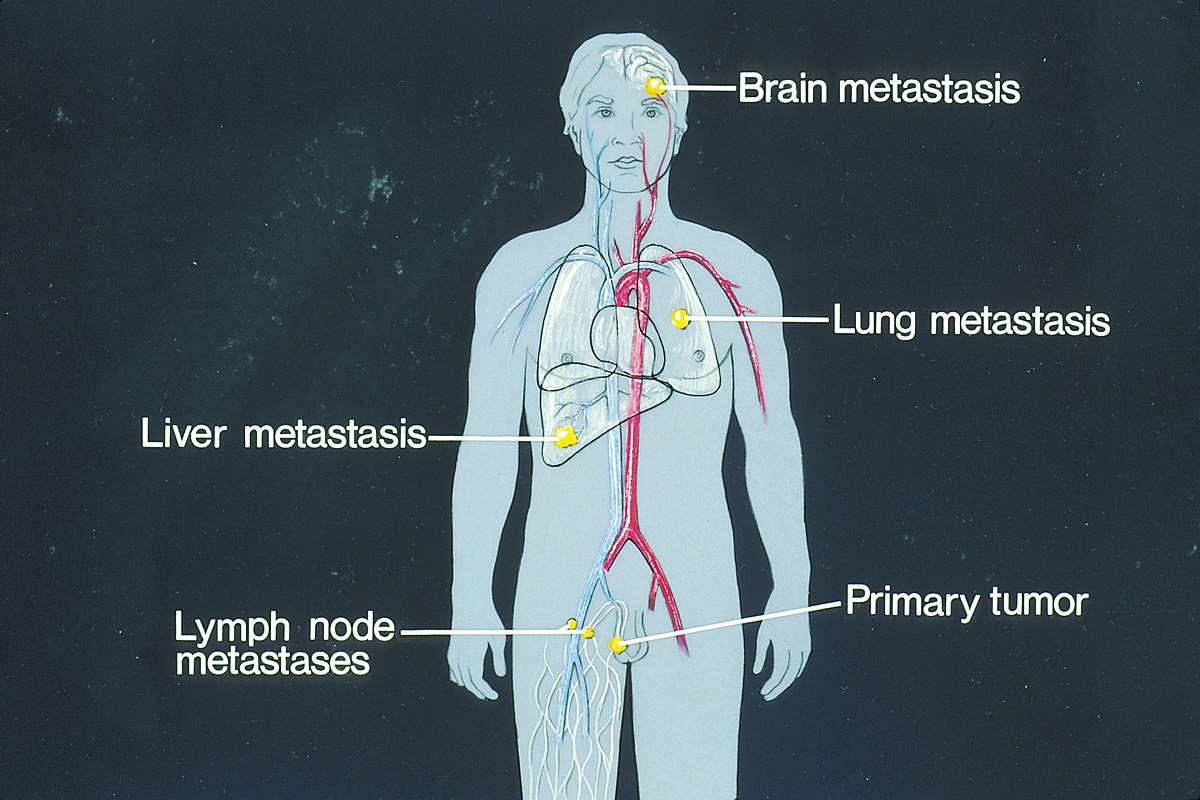
Common sites of metastasis include the following areas[6],[7]:
- Lungs: The lungs are a frequent site of metastasis because they receive a large volume of blood from the body’s circulation. Cancer cells circulating in the bloodstream can easily lodge in the small blood vessels of the lungs, leading to secondary tumor formation.
- Liver: The liver receives a robust blood supply and plays a significant role in blood filtration, making it another common site for metastasis.
- Brain: Cancer cells can reach the brain through the bloodstream or by direct extension from nearby structures.
- Bones: Many cancers, such as breast, lung, and prostate cancer, have a tendency to metastasize to the bones.
- Lymph Nodes: Lymph nodes are part of the lymphatic system and play a role in filtering lymph (a fluid that transports immune cells and waste products). Tumors located in close proximity to the lymph nodes, especially breast, gastrointestinal, urological, gynecological, and some skin cancers, are more likely to spread through the lymphatic system.
- Bowel and Intestines: The bowel and intestines, including the colon and rectum, are susceptible to metastasis, particularly in colorectal cancer. Cancer cells from the colon can also spread to nearby lymph nodes and to the liver.
- Adrenal Glands: The adrenal glands that sit atop the kidneys can be affected by metastatic cancer. The adrenal glands have a rich blood supply, making them potential targets for cancer cells circulating in the bloodstream.
- Skin: The skin is also a common site for cancer metastasis. Melanoma, a type of skin cancer, has a propensity to spread to other parts of the body, including distant skin sites and internal organs.
- Ovaries: In women, ovarian metastasis can occur from cancers that have spread through the bloodstream or lymphatic system. Breast, colorectal, and stomach cancers are examples of cancers that can metastasize to the ovaries.
- Kidneys: Kidney metastasis can occur when cancer cells from other parts of the body reach the kidneys through the bloodstream. The kidneys filter the blood, making them a potential site for cancer cells to become trapped.
Metastasis is complex and involves several distinct steps, each of which contributes to the cancer’s ability to establish secondary tumors in different organs and tissues. Read more details about the process of metastasis in the following box.
A Closer Look: Metastasis[8]
- Local Invasion: The process of metastasis begins with the primary tumor growing and infiltrating nearby tissues. Cancer cells release enzymes that break down proteins surrounding cells. This enzymatic activity allows the cancer cells to break down barriers between healthy tissues, enabling them to spread into surrounding areas.
- Intravasation: As the primary tumor grows, some cancer cells may invade nearby blood or lymphatic vessels. They enter these vessels by breaking through the vessel walls or migrating between cells that line the vessel walls. Once inside, cancer cells can be carried by the bloodstream or lymphatic system to distant sites in the body.
- Circulation: Cancer cells that have entered the bloodstream or lymphatic system are carried throughout the body. They flow with the blood or lymphatic fluid, traveling to various organs and tissues.
- Adhesion: Eventually, cancer cells carried by the bloodstream or lymphatic system come into contact with the blood vessels of other organs. To establish secondary tumors, cancer cells must adhere to the walls of these vessels. They use specific molecules to anchor themselves to the vessel walls at distant sites.
- Extravasation: After adhering to the vessel walls, cancer cells must then cross the vessel walls to enter the surrounding tissue. They do this by squeezing through the vessel walls, a process known as extravasation.
- Microenvironment Adaptation: Once cancer cells have successfully extravasated and entered a new tissue, they encounter a new microenvironment. The cells must adapt to the specific conditions of the tissue they have invaded. Some cancer cells may not survive in this new environment, but those that do can start to proliferate and form a secondary tumor.
- Secondary Tumor Formation: Cancer cells that have successfully adapted to the new microenvironment begin to divide and multiply, forming a secondary tumor at the distant site. These secondary tumors are also referred to as metastatic tumors. Over time, these tumors can grow, invade nearby tissues, and disrupt the normal function of the affected organ.
View a supplementary YouTube video[9] from the National Cancer Institute on how cancer spreads: Metastasis: How Cancer Spreads.
When cancer spreads through metastasis, such as when colon cancer spreads to the liver, it is still medically treated as colon cancer even though the site is the liver.
Etiology of Cancer
Although the causes of many types of cancer remain unknown, there are specific elements that have been identified that enhance the risk of developing cancer. Three primary factors that have been shown to influence cancer development include the following:
- Exposure to Carcinogens: A carcinogen is any substance capable of causing cancer. Carcinogens can come from many different types of sources, including industrial chemicals, tobacco smoke, pollutants, radiation, and viruses. These carcinogenic agents cause damage to cellular DNA, causing mutations within the cell. The mutations disrupt the normal regulatory mechanisms, allowing for abnormal cell growth and division. The extent and duration of exposure, as well as an individual’s susceptibility, contribute to the overall impact of carcinogens in cancer development.[10]
- Genetic Predisposition: Genetic predisposition refers to inherited mutations or variations in certain genes that can increase an individual’s susceptibility to cancer. While not all cancers are directly inherited, certain genetic mutations can elevate the risk of developing specific types of cancer. Mutations in tumor suppressor genes, which regulate cell growth and prevent mutations, and oncogenes, which promote cell growth, are of particular significance. For instance, mutations in the BRCA1 and BRCA2 genes are associated with a higher risk of breast and ovarian cancers. Advances in genetic testing have enabled the identification of individuals at elevated risk in nearly every type of cancer, allowing for targeted monitoring and preventive interventions.[11]
- Immune Function: A healthy functioning immune system is constantly scanning the body for abnormal cells and potential threats. A robust immune response can detect and eliminate cancerous cells before they take significant action. However, many cancer cells develop strategies to evade immune recognition, a phenomenon known as immune evasion. Cancer immunosurveillance is a dynamic process involving various immune cells, such as T cells and natural killer cells, as well as molecules like cytokines. Immunotherapy, a cutting-edge approach, aims to harness the power of the immune system to target and destroy cancer cells. For example, CAR-T cell therapy is an example of innovative treatment that manipulates immune function to combat cancer.[12]
Risk Factors for Cancer
An individual’s cancer risk is impacted by numerous factors that may contribute to the development of the disease. It is important to understand the mechanisms of carcinogenesis, a medical term for the development of cancer.
Risk factors for developing cancers include the following[13]:
- Oncogene Activation: Oncogene activation is a central mechanism in carcinogenesis. Oncogene activation occurs when specific genes within a cell’s normal makeup become replicated out of control due to loss of cellular regulation or exposure to carcinogenic agents. These oncogenes are not inherently abnormal because they are part of every cell’s genetic composition. The problem arises when the activity of oncogenes is not regulated, leading to unrestricted cell growth and division. This unchecked growth forms the basis of cancer development. See Figure 4.12[14] for an illustration of oncogene activation.
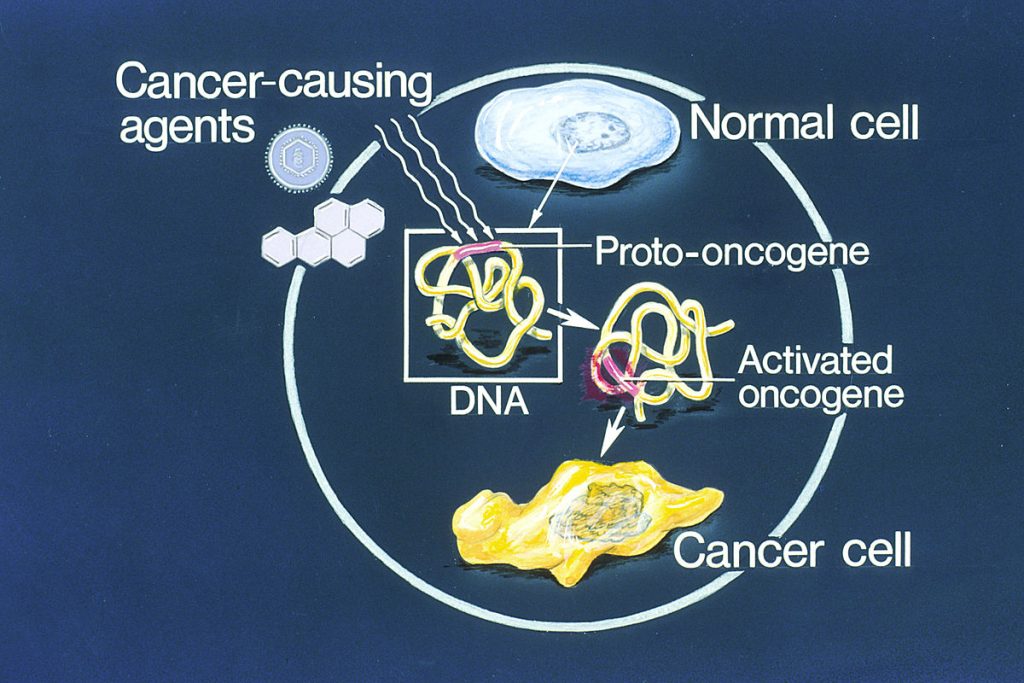
- Chemical Carcinogenesis: Chemical carcinogenesis occurs due to exposure to various chemicals, drugs, and products encountered in everyday life. For example, tobacco is responsible for approximately 30% of cancer diagnoses in North America.[15] Carcinogenic chemicals can disrupt cellular processes and lead to DNA mutations.
- Physical Carcinogenesis: Two physical agents, radiation and chronic irritation, have been identified as contributors to cancer development. Ionizing radiation and ultraviolet (UV) radiation have been associated with DNA damage that can trigger oncogene activation and mutations. Chronic irritation, exemplified by the higher incidence of skin cancer in scar tissues of individuals with burn scars or other severe skin injuries, underscores the importance of cell division and DNA mutation in cancer risk.
- Viral Carcinogenesis: Viral carcinogenesis occurs when viruses infect human cells and disrupt the DNA strands. By inserting their genetic material into the host’s DNA, these viruses can manipulate cellular processes and promote oncogene activation. Viral infections, such as human papillomavirus (HPV) and hepatitis B and C, have been linked to various cancers.
- Dietary Factors: Dietary habits play a role in cancer risk. Suspected factors include low fiber intake and high consumption of red meat and animal fats. Furthermore, additives, preservatives, and high-heat or grilling cooking methods may exert cancer-promoting effects.
- Immune Function and Age: The immune system plays a critical role in detecting and eliminating cancerous cells. Reduced immune function, often seen in immunosuppressed individuals, can increase cancer susceptibility. Age is also a significant risk factor for individuals over 60 due to a gradual decline in immune function and cellular repair mechanisms.
- Genetic Risk: Genetic predisposition, identified through genetic testing, can provide valuable insights into an individual’s susceptibility to certain types of cancer.
Prevention of Cancer
Prevention strategies are important for reducing cancer risk and optimizing client outcomes. Prevention strategies include primary, secondary, and tertiary prevention strategies.
Primary Prevention
Primary prevention involves strategies aimed at preventing the initial occurrence of cancer.[16],[17] These strategies focus on reducing exposure to risk factors and promoting healthy behaviors to minimize the likelihood of cancer development.
Several key strategies fall under primary prevention[18],[19]:
- Avoid Known or Potential Carcinogens: This involves identifying and avoiding substances or environmental factors known to increase the risk of cancer. For example, individuals are encouraged to avoid tobacco smoke, limit exposure to harmful chemicals, and adopt protective behaviors like using sunscreen to reduce the risk of skin cancer.
- Modify Associated Factors: Lifestyle modifications play a crucial role in primary prevention. Encouraging a balanced diet rich in fruits, vegetables, and whole grains, along with regular physical activity, can help reduce the risk of various cancers. Limiting alcohol consumption and maintaining a healthy weight are also important factors in cancer prevention.
- Remove “At-Risk” Tissues: Some individuals with precancerous conditions or high-risk genetic profiles might choose to undergo prophylactic surgeries to remove tissues that have a high likelihood of developing cancer. For example, individuals with certain genetic mutations linked to breast cancer might opt for preventive mastectomy to reduce their risk.
- Vaccinate: Certain vaccines can prevent infections that are strongly associated with cancer development. For example, the human papillomavirus (HPV) vaccine can significantly reduce the risk of cervical and HPV-related cancers in females and males. In addition, hepatitis B vaccination lowers the risk of developing liver cancer.
Secondary Prevention
Secondary prevention focuses on early detection and intervention to identify cancer at an early stage, when it is more treatable.[20],[21] Screening strategies are key components of secondary prevention and include the following:
- Screening Programs: Regular screening tests aim to detect cancer before symptoms appear. Mammograms, Pap tests, colonoscopies, and prostate-specific antigen (PSA) tests are examples of screening tests that aid in the early detection.[22]
- Early Diagnosis: When cancer is detected through screening, it is often at an earlier stage, when treatment outcomes are better. Early diagnosis allows for more conservative treatment options, potentially avoiding more aggressive interventions like surgery, chemotherapy, or radiation therapy.
- Reduced Mortality: Successful secondary prevention can lead to reduced cancer-related mortality rates. By identifying and treating cancer in its early stages, the chances of successful treatment and long-term survival are significantly improved.
- Improved Quality of Life: Early detection and treatment can also lead to better overall quality of life for cancer survivors, as the need for extensive treatments and their associated side effects may be minimized.[23],[24]
Tertiary Prevention
The goal of tertiary prevention in someone who is diagnosed with cancer is to manage symptoms, enhance quality of life, and reduce the risk of complications.[25],[26]
Some examples of tertiary prevention are as follows[27],[28]:
- Providing antiemetics to a client who has nausea and vomiting due to chemotherapy
- Obtaining a referral for palliative care
- Obtaining a referral to a rehabilitation center post-hospitalization for cancer-related issues
- Administering chemotherapy to prevent the metastasis of a primary tumor
- Referring the client to a support group consisting of others suffering from similar issues
- Educating the client on potential complications of their disorder, how to avoid complications, and what symptoms should be reported to their provider
Nursing Role in Prevention Strategies
Nurses are important in helping to ensure individuals participate in both primary and secondary prevention strategies. They have a critical role for providing client education regarding risk factors and surveillance strategies.
For example, nurses teach mnemonics such as “CAUTION” to help individuals become aware of early signs of cancer. These warning signs of cancer are as follows[29]:
C: Changes in bowel or bladder habits
A: A sore that does not heal
U: Unusual bleeding or discharge
T: Thickening or lump in the breast or elsewhere
I: Indigestion or difficulty swallowing
O: Obvious change in a wart or mole
N: Nagging cough or hoarseness
Types of Cancer
There are various types of cancers that originate in specific tissues or organs, with specific characteristics and behaviors. Cancer falls into two main categories: solid tumors and hematological malignancies. Some cancers grow slowly, while others are more aggressive. Table 4.3a provides an overview of major types of cancers, their descriptions, common risk factors, screening methods, and treatment options. It is important to remember that individual cases can vary widely, and health care providers offer personalized guidance regarding cancer prevention, detection, and treatment.
Table 4.3a. Types of Cancer[30],[31],[32],[33]
| Cancer Type | Description | Common Risk Factors | Screening and Diagnostic Tests | Treatment Options |
|---|---|---|---|---|
| Breast Cancer | Affects breast tissue, commonly in women but can occur in men | Gender, Age, and Family History | Mammography | Surgery, Radiation, Chemotherapy, Targeted Therapy, and Hormonal Therapy |
| Lung Cancer | Develops in lung tissues and often linked to smoking or exposure to harmful substances | Smoking and Radon Exposure | CT Scans and Sputum Tests | Surgery, Radiation, Chemotherapy, and Targeted Therapies |
| Prostate Cancer | Occurs in the prostate gland of men, usually slow-growing and common in older men | Age and Family History | PSA Blood Test | Surgery, Radiation, Chemotherapy, Targeted Therapy, and Hormone Therapy |
| Colorectal Cancer | Affects the colon or rectum, commonly adenocarcinomas | Age, Diet, and Family History | Colonoscopy | Surgery, Chemotherapy, Radiation, and Targeted Therapies |
| Skin Cancer | Develops in skin cells due to exposure to UV radiation from the sun or tanning beds | Sun Exposure and Fair Skin | Skin Exams | Surgery, Radiation, Immunotherapy, Targeted Therapy, and Topical Chemotherapy |
| Leukemia (Lymphocytic Cancer) | Blood cancer that affects bone marrow and blood cells | Genetic Factors | Blood Tests | Chemotherapy, Stem Cell Transplant, and Immunotherapy |
| Lymphoma | Cancer of the lymphatic system, includes Hodgkin and non-Hodgkin lymphomas | Immune System Disorders | Biopsy and Imaging | Chemotherapy, Radiation, and Immunotherapy |
| Ovarian Cancer | Occurs in the ovaries of women, often diagnosed at an advanced stage | Family History and Age | Pelvic Exams and Imaging | Surgery, Radiation, Chemotherapy, and Targeted Therapies |
| Pancreatic Cancer | Develops in the pancreas, often diagnosed at an advanced stage | Smoking and Obesity | Imaging and Biopsy | Surgery, Chemotherapy, and Radiation |
| Brain Cancer | Affects brain cells | Radiation Exposure and Family History | Imaging and Biopsy | Surgery, Radiation, and Chemotherapy |
| Myeloid Cancers | A group of blood cancers affecting myeloid cells in bone marrow, such as Acute Myeloid Leukemia, Chronic Myeloid Leukemia, Multiple Myeloma, etc. | Genetic Mutations | Blood Tests and Biopsy | Chemotherapy, Targeted Therapy, and Stem Cell Transplant |
Staging
Staging of cancer is completed by a provider to determine how big a tumor is and whether or not it has metastasized to other parts of the body. This allows providers to tailor cancer treatment according to its stage.
There are a variety of ways to stage cancer, but two common staging modalities are the TNM system and stage grouping.
TNM System
- T: Tumor Size
- N: Number of lymph nodes involved
- M: If metastasis has occurred
With this staging system, there are numbers that follow each of the letters that provide more detail about tumor size, the number of lymph nodes involved, and if metastasis has occurred. For example, a tumor designated as “T1” is a smaller tumor than a tumor designated “T4.” In regard to lymph nodes, “N0” means there is no cancer present in adjacent lymph nodes, whereas “N3” indicates multiple nearby lymph nodes have cancer present. For metastasis, “M0” means no metastasis has occurred, whereas “M1” indicates cancer has spread to distant parts of the body.[34]
Stage Grouping
Cancerous tumors can also be assigned an overall stage with stage grouping. With this staging system, tumors are designated Stage 0 to Stage IV. Stage 0 is also known as carcinoma in situ. This means that cancerous or abnormal cells are present, but they are localized to the initial layer of cells where they were first discovered. Stages I-III means that cancer exists, and the higher the number, the bigger the tumor is and the more it has invaded adjacent tissues. Stage IV cancer means that the cancer has spread to remote parts of the body. Generally, the lower the stage upon diagnosis, the better chance the client has for achieving a cure or entering remission.[35],[36]
Oncological Emergencies
Oncological emergencies encompass a variety of clinical conditions that arise acutely in the client with cancer. These emergencies can be related to metabolic changes in the body that are associated with cancer, structural changes in which a cancerous tumor is impinging on other organs or structures, or a result of cancer treatment.[37],[38],[39] See Table 4.3b for common oncological emergencies, related signs and symptoms, and treatment options.
Table 4.3b. Oncological Emergencies[40],[41],[42]
| Oncological Emergency | Signs and Symptoms | Treatment |
|---|---|---|
| Hypercalcemia: Increased calcium levels caused by tumor invasion into bone or increased production of parathyroid hormone or vitamin D3. | Serum calcium level greater than 10.5 mg/dL
Changes in cognition Weakness Loss of appetite, nausea, vomiting, constipation, or increased thirst Coma Increased urination Irregular heartbeat |
Monitor albumin levels as the majority of calcium is protein bound
Aggressive hydration Loop diuretics Bisphosphonates and/or calcitonin Steroids (if the cause of elevated calcium is due to overproduction of vitamin D3) Hemodialysis may be required Electrocardiogram |
| Tumor Lysis Syndrome: Tumor cells break down in response to cancer treatment and release intracellular contents into the bloodstream. Can also occur spontaneously in some cancers. This disrupts the normal balance of electrolytes in the body. | Increased serum levels of uric acid, phosphorus, and potassium
Decreased levels of calcium Signs of kidney failure or elevated BUN and creatinine Irregular heartbeat Seizures Fatigue Nausea and vomiting |
Electrocardiogram
Aggressive hydration Monitor urine output Decrease intake of foods containing phosphorus and potassium Hemodialysis may be required Phosphate binders Calcium supplementation Rasburicase to decrease uric acid levels |
| Superior Vena Cava Syndrome: Compression of the superior vena cava by a cancerous tumor. | Edema of the face, neck, or upper extremities
Jugular vein distention Cough, shortness of breath at rest, or hoarse voice Chest/shoulder pain |
Radiation or chemotherapy to reduce the tumor size
Steroids Stenting of the superior vena cava to prevent compression |
| Syndrome of Inappropriate Antidiuretic Hormone (SIADH): When cancerous tumors produce excessive antidiuretic hormone. Can also be caused by some chemotherapy medications. Leads to water retention. | Low sodium levels
Decreased osmolarity of the blood Concentrated urine Nausea, vomiting, or constipation Weakness |
Fluid restriction of 500 to 1000 mL/day
Hypertonic saline may be used Monitor urine output |
| Extravasation of Chemotherapy Medications: When chemotherapy seeps into surrounding tissues instead of going into the bloodstream. | Pain, edema, or redness at site of extravasation
Blister formation and necrosis at site of extravasation |
Halt the infusion as soon as extravasation is noted
Aspirate any remaining drug from cannula Leave cannula of access device in place until plan of action is determined (plan will vary based on particular medication) Cold or warm compresses (approach depends on particular medication) Give antidote if available Elevate affected limb |
General Medical Interventions for Cancer
Cancer treatment commonly involves a combination of many different therapies in order to optimize client outcomes. Medical interventions continue to evolve with advancements in technology and emerging medical research. Surgical and medical interventions offer specific approaches to cancer treatment, respective of the type and stage of cancer, the client’s overall health, and the treatment goals.
Surgery
Surgery is a common treatment option for various types of cancer. It involves the physical removal of cancerous tissues from the body. Surgical interventions can serve multiple purposes in cancer care.[43] See Table 4.3c for examples of common surgeries performed for cancer.
Table 4.3c. Examples of Surgeries Performed For Cancer[44]
| Surgery Type | Description | Example |
|---|---|---|
| Prophylaxis Surgery | Removes at-risk tissue to prevent cancer development. | Removing a benign mole from a sun-exposed area. |
| Diagnosis Surgery | Removes suspected lesion for examination and testing. | Biopsy of a suspicious lump. |
| Curative Surgery | Focuses on complete removal of all cancerous tissue. | Surgical removal of a localized tumor. |
| Control Surgery | Focuses on partial removal of tumor to increase efficacy of other treatments. | Debulking surgery to reduce tumor size. |
| Palliative Surgery | Aims to improve quality of life by alleviating symptoms. | Removing a tumor causing pain. |
| Reconstruction/Rehabilitation Surgery | Enhances function and appearance post-cancer treatment. | Breast reconstruction after mastectomy. |
Radiation
Radiation therapy uses high-energy rays or particles to target and damage cancer cells. This treatment can be systemic or localized, meaning it aims to destroy or shrink tumors while minimizing damage to surrounding healthy tissue.[45]
It can serve to cure, control, or relieve symptoms of the disease by damaging cancer cells through exposure to ionizing radiation.
When cells are subjected to ionizing radiation, the particles within the cell’s nucleus undergo rearrangement, resulting in the release of a substantial amount of intracellular energy.[46] The energy emitted by radioactive elements can vary in its ability to penetrate tissues and inflict damage to cells. See Figure 4.13[47] for an image of a client undergoing radiation therapy.
Radiation therapy has localized effects, impacting tissues within the radiation path, rather than systemic effects associated with chemotherapy. For instance, when treating lung cancer with radiation to the chest area, changes like skin alterations and hair loss are observed solely within the chest region subjected to radiation. This localized targeting is a key advantage of radiation therapy.
There is a limit to the amount of radiation an area of the body can safely receive over the course of an individual’s lifetime. Depending on how much radiation an area has already been treated with, a client may be able to have radiation therapy to that area a second time. But, if one area of the body has already received the safe lifetime dose of radiation, another area might still be treated if the distance between the two areas is large enough.[48]
There are different methods for delivering radiation therapy, including teletherapy and brachytherapy.
- External beam radiation therapy: Teletherapy, or external beam radiation therapy, comes from a machine that aims radiation at the client’s cancer. The machine is large, and it may be noisy. The machine does not touch the client, but can move around them, sending radiation to the cancer from many directions. External beam radiation therapy is a local treatment, which means it treats a specific part of the body. For example, if the client has lung cancer, radiation is only provided to the chest, not the whole body.[49]
- Internal radiation therapy: Internal radiation therapy is a treatment in which a source of radiation is put inside the body near the tumor site. It is often used for specific cancers like cervical, prostate, or thyroid cancer. The radiation source can be solid or liquid. Internal radiation therapy with a solid source is called brachytherapy. In this type of treatment, seeds, ribbons, or capsules that contain a radiation source are placed near the tumor and will either remain in place to deliver radiation over a period of time or be removed. Like external beam radiation therapy, brachytherapy is a local treatment and treats only a specific part of your body. Clients undergoing brachytherapy emit radiation for a certain period, creating a potential exposure hazard to others during that time and requiring specific precautions.[50],[51] For example, soluble isotopes can be ingested or injected to treat thyroid cancer. These isotopes are eventually eliminated through the individual’s urine and stool, causing a radioactive risk to others. These radioactive wastes should not be handled directly by nurses or family members and must be managed according to agency policy. After the isotope is fully eliminated from the client’s body, neither the client nor their waste products pose a radioactive risk.
Chemotherapy
Chemotherapy involves the use of drugs to destroy or slow the growth of cancer cells.[52] Chemotherapy treatment employs chemical agents to kill cancer cells and increase the individual’s survival. Chemotherapy drugs can be administered orally, subcutaneously, intramuscularly, or intravenously. They enter the bloodstream and kill cancer cells throughout the body.
Chemotherapy has cytotoxic effects within the body, meaning it impacts all cells that are rapidly dividing.[53] This effect is important for killing cancer cells but also impacts other cells that divide rapidly, such as those in hair follicles. This is why many individuals being treated with certain types of chemotherapy may experience hair loss. The goal of chemotherapy is to find balance between eradicating cancer cells and minimizing harm to normal tissues. Providers closely monitor the dose and impact of chemotherapy agents, titrating the medication to help achieve a desired effect while minimizing harmful side effects.
Chemotherapy is used for these purposes[54]:
- Adjuvant Chemotherapy: Given after surgery or radiation therapy to kill any remaining cancer cells and lower the risk of recurrence.
- Neoadjuvant Chemotherapy: Administered before surgery or radiation to shrink tumors and make them more manageable for surgical removal or radiation treatment.
- Systemic Chemotherapy: Used to treat cancers that have metastasized to other parts of the body. It is effective against rapidly dividing cells.
Because of the potential risks associated with exposure to chemotherapy agents, only specifically trained or oncology certified nurses (OCN) administer chemotherapy, based on agency policy. Oncology certified nurses provide specialized care for clients with cancer after completing additional courses and successfully passing a certification exam. Nurses who administer chemotherapy follow specific protocols and guidelines when handling chemotherapy or clients’ urine and stool to decrease potential risks associated with exposure to these hazardous substances.
Personal protective equipment (PPE) is a critical component of ensuring nurse safety related to chemotherapy. PPE helps create a barrier between the nurse and the hazardous substances, preventing direct contact and reducing the potential for exposure. The use of PPE is guided by established guidelines from organizations such as the Occupational Safety & Health Administration (OSHA) and the Oncology Nursing Society (ONS).
Specific types of PPE used to enhance nurse safety related to the administration of chemotherapy are as follows:
- Eye Protection: Goggles or face shields prevent exposure to potentially harmful chemicals and aerosolized particles. This protects the eyes from accidental splashes or airborne contaminants.
- Masks: Masks are worn to protect the respiratory system from inhaling airborne particles or droplets that may contain chemotherapy drug residues.
- Nitrile Gloves: Nitrile gloves are made of synthetic rubber that resists punctures and harsh chemicals and add an extra layer of protection to the hands.
- Gown: Protective gowns are worn to cover clothing and minimize the risk of contamination. These gowns act as a barrier against direct contact with chemotherapy drugs from clients who are receiving chemotherapy.
In addition to PPE, nurses are trained in proper handling techniques of chemotherapy, spill management, waste disposal, and decontamination procedures. These guidelines ensure that nurses have the knowledge and tools needed to minimize their risks associated with exposure to chemotherapy drugs.
Additional Therapies
Additional types of cancer treatment mobilize the body’s own defense mechanisms and target specific cancer cells, including immunotherapy, targeted therapy, photodynamic therapy, hormonal therapy, and vaccine therapy:
- Immunotherapy: Immunotherapy boosts the body’s immune system to identify and attack cancer cells. It can include immune checkpoint inhibitors, cancer vaccines, and adoptive T-cell therapy.[55]
- Targeted Therapy: Targeted therapy targets specific molecules or genetic alterations that drive cancer growth. It aims to disrupt specific pathways involved in cancer development. For example, imatinib is a medication used as targeted therapy to inhibit a specific molecular target that is overactive in leukemias.[56]
- Photodynamic Therapy: Photodynamic therapy involves a photosensitizing agent and light to destroy cancer cells. It’s used primarily for skin, lung, and esophageal cancers.[57]
- Hormonal Therapy: Hormonal therapy is used to treat hormone-sensitive cancers like breast and prostate cancer. It involves blocking or lowering the levels of hormones that promote cancer growth. For example, tamoxifen, a selective estrogen receptor modulator (SERM), is commonly used to treat hormone receptor-positive breast cancer.[58]
- Vaccine Therapy: Cancer vaccines are directed against the cells of one’s own body. Treated cancer cells are injected into clients with cancer to enhance their immune response against cancer and prolong survival. The immune system has the capability to detect these cancer cells and proliferate faster than the cancer cells do, thus overwhelming the cancer in a similar way as they do for viruses. Cancer vaccines are being developed for malignant melanoma and renal (kidney) cell carcinoma.[59]
Bone Marrow Transplantation & Stem Cell Treatment
Bone marrow transplantation and stem cell treatment are advanced medical interventions that have revolutionized cancer treatment. These procedures harness the remarkable regenerative potential of stem cells.
Bone marrow transplantation involves the replacement of damaged or diseased bone marrow with healthy stem cells to restore the body’s ability to produce essential blood cells.[60] The bone marrow plays a crucial role in generating red blood cells, white blood cells, and platelets. In cancer cases, such as leukemia, lymphoma, and multiple myeloma, the bone marrow’s ability to produce healthy blood cells is compromised due to the aggressive nature of these diseases or the effects of chemotherapy and radiation treatments.[61]
During a bone marrow transplant, a client receives either their own healthy stem cells (autologous transplant) or those from a compatible donor (allogeneic transplant). Autologous transplants are typically used when the client’s own stem cells are collected, purified, and then reinfused following intensive chemotherapy or radiation to destroy cancer cells. Allogeneic transplants involve obtaining stem cells from a matching donor, often a sibling or unrelated donor, and then transplanting them into the client after preparatory treatments. These transplanted stem cells migrate to the bone marrow and begin producing new, healthy blood cells, aiding in the recovery of the client’s immune system and blood cell count.[62]
Stem cell treatment uses stem cells to replace damaged tissues, enhance the body’s natural healing processes, and combat cancerous growth.[63] Stem cells are undifferentiated cells with the unique ability to differentiate into different cell types, making them invaluable for regenerative medicine. Stem cell treatment may involve using a client’s own stem cells or donor-derived stem cells to rebuild tissues damaged by cancer or its treatments.[64],[65]
- “Tumor_Types_MTK.jpg” by WolfpackBME is licensed under CC BY-SA 4.0 ↵
- National Cancer Institute. (n.d.). Cancer terms. National Institutes of Health. https://training.seer.cancer.gov/disease/cancer/terms.html ↵
- National Cancer Institute. (n.d.). Cancer terms. National Institutes of Health. https://training.seer.cancer.gov/disease/cancer/terms.html ↵
- Patel, A. (2020). Benign vs malignant tumors. JAMA Oncology, 6(9), 1488. https://jamanetwork.com/journals/jamaoncology/fullarticle/2768634 ↵
- “Sites_of_potential_metastases_illustration.jpg” by unknown author, via National Cancer Institute is licensed in the Public Domain. ↵
- Patel, A. (2020). Benign vs malignant tumors. JAMA Oncology, 6(9), 1488. https://jamanetwork.com/journals/jamaoncology/fullarticle/2768634 ↵
- National Cancer Institute. (2020). Metastatic cancer: When cancer spreads. National Institutes of Health. https://www.cancer.gov/types/metastatic-cancer ↵
- National Cancer Institute. (2020). Metastatic cancer: When cancer spreads. National Institutes of Health. https://www.cancer.gov/types/metastatic-cancer ↵
- National Cancer Institute. (2017, February 12). Metastasis: How cancer spreads [Video]. YouTube. All rights reserved. https://www.youtube.com/watch?v=fQwar_-QdiQ ↵
- National Cancer Institute. (n.d.). Cancer risk factors. National Institutes of Health. https://training.seer.cancer.gov/disease/cancer/risk.html ↵
- National Cancer Institute. (n.d.). Cancer risk factors. National Institutes of Health. https://training.seer.cancer.gov/disease/cancer/risk.html ↵
- National Cancer Institute. (n.d.). Cancer risk factors. National Institutes of Health. https://training.seer.cancer.gov/disease/cancer/risk.html ↵
- National Cancer Institute. (n.d.). Cancer risk factors. National Institutes of Health. https://training.seer.cancer.gov/disease/cancer/risk.html ↵
- “Oncogenes_illustration.jpg” by unknown author, via National Cancer Institute is licensed in the Public Domain. ↵
- National Cancer Institute. (n.d.). Cancer risk factors. National Institutes of Health. https://training.seer.cancer.gov/disease/cancer/risk.html ↵
- Colditz, G. A. (2022). Overview of cancer prevention. UpToDate. https://www.wolterskluwer.com/en/solutions/uptodate ↵
- Fletcher, G. S. (2022). Evidence-based approach to prevention. UpToDate. https://www.wolterskluwer.com/en/solutions/uptodate ↵
- Colditz, G. A. (2022). Overview of cancer prevention. UpToDate. https://www.wolterskluwer.com/en/solutions/uptodate ↵
- Fletcher, G. S. (2022). Evidence-based approach to prevention. UpToDate. https://www.wolterskluwer.com/en/solutions/uptodate ↵
- Colditz, G. A. (2022). Overview of cancer prevention. UpToDate. https://www.wolterskluwer.com/en/solutions/uptodate ↵
- Fletcher, G. S. (2022). Evidence-based approach to prevention. UpToDate. https://www.wolterskluwer.com/en/solutions/uptodate ↵
- Cancer Research UK. (n.d.). Tests and scans. https://www.cancerresearchuk.org/about-cancer/tests-and-scans ↵
- Colditz, G. A. (2022). Overview of cancer prevention. UpToDate. https://www.wolterskluwer.com/en/solutions/uptodate ↵
- Fletcher, G. S. (2022). Evidence-based approach to prevention. UpToDate. https://www.wolterskluwer.com/en/solutions/uptodate ↵
- Loomans-Kropp, H. A. & Umar, A. (2019). Cancer prevention and screening: The next step in the era of precision medicine. npj Precision Oncology, 3. https://doi.org/10.1038/s41698-018-0075-9 ↵
- Kisling, L.A., & Das, J.M. (2023). Prevention Strategies. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK537222/ ↵
- Loomans-Kropp, H. A. & Umar, A. (2019). Cancer prevention and screening: The next step in the era of precision medicine. npj Precision Oncology, 3. https://doi.org/10.1038/s41698-018-0075-9 ↵
- Kisling, L.A., & Das, J.M. (2023). Prevention Strategies. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK537222/ ↵
- Cunha, J. P. (2022). What are the seven warning signs of cancer? eMedicineHealth. https://www.emedicinehealth.com/what_are_the_seven_warning_signs_of_cancer_caution/article_em.htm ↵
- Cancer Research UK. (n.d.). Your cancer type. https://www.cancerresearchuk.org/about-cancer/type ↵
- Cancer Research UK. (2021). Treatment for cancer. https://www.cancerresearchuk.org/about-cancer/treatment ↵
- Cancer Research UK. (n.d.). Tests and scans. https://www.cancerresearchuk.org/about-cancer/tests-and-scans ↵
- National Cancer Institute. (n.d.). Cancer terms. National Institutes of Health. https://training.seer.cancer.gov/disease/cancer/terms.html ↵
- National Cancer Institute. (2022). Cancer staging. National Institutes of Health. https://www.cancer.gov/about-cancer/diagnosis-staging/staging ↵
- National Cancer Institute. (2022). Cancer staging. National Institutes of Health. https://www.cancer.gov/about-cancer/diagnosis-staging/staging ↵
- National Cancer Institute. (2022). Cancer staging. National Institutes of Health. https://www.cancer.gov/about-cancer/diagnosis-staging/staging ↵
- Higdon, M. L., Atkinson, C. J., & Lawrence, K. V. (2018). Oncological emergencies: Recognition and initial management. American Family Physician, 97(11), 741-748. https://www.aafp.org/pubs/afp/issues/2018/0601/p741.htm ↵
- Klemencic, S., & Perkins, J. (2019). Diagnosis and management of oncologic emergencies. The Western Journal of Emergency Medicine, 20(2), 316–322. https://doi.org/10.5811/westjem.2018.12.37335 ↵
- Kim, J. T., Park, J. Y., Lee, H. J., & Cheon, Y. J. (2020). Guidelines for the management of extravasation. Journal of Educational Evaluation for Health Professions, 17, 21. https://doi.org/10.3352/jeehp.2020.17.21 ↵
- Higdon, M. L., Atkinson, C. J., & Lawrence, K. V. (2018). Oncological emergencies: Recognition and initial management. American Family Physician, 97(11), 741-748. https://www.aafp.org/pubs/afp/issues/2018/0601/p741.htm ↵
- Klemencic, S., & Perkins, J. (2019). Diagnosis and management of oncologic emergencies. The Western Journal of Emergency Medicine, 20(2), 316–322. https://doi.org/10.5811/westjem.2018.12.37335 ↵
- Kim, J. T., Park, J. Y., Lee, H. J., & Cheon, Y. J. (2020). Guidelines for the management of extravasation. Journal of Educational Evaluation for Health Professions, 17, 21. https://doi.org/10.3352/jeehp.2020.17.21 ↵
- Cancer Research UK. (2021). Treatment for cancer. https://www.cancerresearchuk.org/about-cancer/treatment ↵
- Cancer Research UK. (2021). Treatment for cancer. https://www.cancerresearchuk.org/about-cancer/treatment ↵
- National Cancer Institute. (2019). Radiation therapy to treat cancer. National Institutes of Health. https://www.cancer.gov/about-cancer/treatment/types/radiation-therapy ↵
- Mitin, T. (2023). Radiation therapy techniques in cancer treatment. UpToDate. https://www.wolterskluwer.com/en/solutions/uptodate ↵
- “Radiation_therapy_for_cancer.jpg” by Jakembradford is licensed under CC BY-SA 4.0 ↵
- National Cancer Institute. (2019). Radiation therapy to treat cancer. National Institutes of Health. https://www.cancer.gov/about-cancer/treatment/types/radiation-therapy ↵
- National Cancer Institute. (2019). Radiation therapy to treat cancer. National Institutes of Health. https://www.cancer.gov/about-cancer/treatment/types/radiation-therapy ↵
- Mitin, T. (2023). Radiation therapy techniques in cancer treatment. UpToDate. https://www.wolterskluwer.com/en/solutions/uptodate ↵
- National Cancer Institute. (2019). Radiation therapy to treat cancer. National Institutes of Health. https://www.cancer.gov/about-cancer/treatment/types/radiation-therapy ↵
- Lichtman, S. (2021). Systemic chemotherapy for cancer in older adults. UpToDate. https://www.wolterskluwer.com/en/solutions/uptodate ↵
- Lichtman, S. (2021). Systemic chemotherapy for cancer in older adults. UpToDate. https://www.wolterskluwer.com/en/solutions/uptodate ↵
- Lichtman, S. (2021). Systemic chemotherapy for cancer in older adults. UpToDate. https://www.wolterskluwer.com/en/solutions/uptodate ↵
- Shoushtari, A. N. (2023). Principles of cancer immunotherapy. UpToDate. https://www.wolterskluwer.com/en/solutions/uptodate ↵
- Atkins, M. B. (2023). Overview of the treatment of renal cell carcinoma. UpToDate. https://www.wolterskluwer.com/en/solutions/uptodate ↵
- Maytin, E. V., & Warren, C. B. (2022). Photodynamic therapy. UpToDate. https://www.wolterskluwer.com/en/solutions/uptodate ↵
- Pritchard, K. I. (2023). Adjuvant endocrine and targeted therapy for postmenopausal women with hormone receptor-positive breast cancer. UpToDate. https://www.wolterskluwer.com/en/solutions/uptodate ↵
- Cancer Research UK. (2021). Treatment for cancer. https://www.cancerresearchuk.org/about-cancer/treatment ↵
- Cancer Research UK. (2021). Treatment for cancer. https://www.cancerresearchuk.org/about-cancer/treatment ↵
- American Cancer Society. (2020). How stem cell and bone marrow transplants are used to treat cancer. https://www.cancer.org/ ↵
- American Cancer Society. (2020). How stem cell and bone marrow transplants are used to treat cancer. https://www.cancer.org/ ↵
- National Cancer Institute. (2015). Stem cell transplants in cancer treatment. National Institutes of Health. https://www.cancer.gov/about-cancer/treatment/types/stem-cell-transplant ↵
- American Cancer Society. (2020). How stem cell and bone marrow transplants are used to treat cancer. https://www.cancer.org/ ↵
- National Cancer Institute. (2015). Stem cell transplants in cancer treatment. National Institutes of Health. https://www.cancer.gov/about-cancer/treatment/types/stem-cell-transplant ↵
Abnormal growths that grow and divide in a controlled manner and do not spread outside their location because they are encapsulated.
Cancerous developments within the body.
Spread of cells through the blood or lymphatic system.
A process by which cancer cells cross into surrounding tissues by squeezing through vessel walls.
Any substance capable of causing cancer.
Genes that regulate cell growth and prevent mutations.
Genes that promote cell growth.
Strategies to evade immune recognition.
A dynamic process involving T cells, natural killer cells, and cytokines to target and destroy cancer cells.
The development of cancer.
Occurs when specific genes within a cell's normal makeup become replicated out of control due to loss of cellular regulation or exposure to carcinogenic agents.
Occurs due to exposure to various chemicals, drugs, and products encountered in everyday life.
Strategies aimed at preventing the initial occurrence of cancer.
Early detection and intervention to identify cancer at an early stage, when it is more treatable.
A type of prevention that focuses on managing symptoms, enhancing the quality of life, and reducing the risk of complications for someone who is diagnosed with cancer.
Completed by a provider to determine how big a tumor is and whether or not it has metastasized to other parts of the body.
Cancerous or abnormal cells are present, but they are localized to the initial layer of cells where they were first discovered.
Encompass a variety of clinical conditions that arise acutely in the client with cancer.
Increased calcium levels caused by tumor invasion into bone or increased production of parathyroid hormone or Vitamin D3.
Tumor cells break down in response to cancer treatment and release intracellular contents into the bloodstream.
Compression of the superior vena cava by a cancerous tumor.
When cancerous tumors produce excessive antidiuretic hormone.
Focuses on complete removal of all cancerous tissue.
Surgery which aims to improve quality of life by alleviating symptoms.
Uses high-energy rays or particles to target and damage cancer cells
Therapy involving directing radiation beams from outside the body toward the tumor.
A procedure where radioactive materials are placed directly inside or very close to the tumor.
The use of drugs to destroy or slow the growth of cancer cells.
Effects within the body that impact cells that are rapidly dividing.
Given after surgery or radiation therapy to kill any remaining cancer cells and lower the risk of recurrence.
Medication administered before surgery or radiation to shrink tumors and make them more manageable for surgical removal or radiation treatment.
Medications used to treat cancers that have spread to distant parts of the body that are effective against rapidly dividing cells.
Helps create a barrier between the nurse and the hazardous substances, preventing direct contact and reducing the potential for exposure
Boosts the body's immune system to identify and attack cancer cells.
Involves the replacement of damaged or diseased bone marrow with healthy stem cells to restore the body's ability to produce essential blood cells.
Typically used when the client's own stem cells are collected, purified, and then reinfused following intensive chemotherapy or radiation to destroy cancer cells.
Involve obtaining stem cells from a matching donor, often a sibling or unrelated donor, and then transplanting them into the client after preparatory treatments.
Uses stem cells to replace damaged tissues, enhance the body's natural healing processes, and combat cancerous growth.
Undifferentiated cells with the unique ability to differentiate into different cell types, making them invaluable for regenerative medicine.

