3.2 Review of Hematology Anatomy & Physiology
The hematological system involves many multifaceted processes that help the body maintain homeostasis and ensure appropriate functioning.
Bone Marrow
Blood formation takes place primarily in the red bone marrow, which plays a central role in producing the key blood components of red blood cells (erythrocytes), white blood cells (leukocytes), and platelets (thrombocytes). Over time, red marrow is replaced by yellow marrow in some bones. Yellow marrow consists mainly of fat cells.
Bone marrow is located in flat bones such as the sternum, skull, and pelvis. In the bone marrow, blood stem cells that are immature and undifferentiated have the remarkable capacity to become any type of cell. As these stem cells differentiate, they require specific growth factors for specialization. For example, erythropoietin, produced by the kidneys in response to hypoxia, is essential for red blood cell (RBC) production. RBC production also relies on adequate levels of iron, vitamin B12, folic acid, copper, and cobalt. Diets that are depleted in these micronutrients impede red blood cell production.
Thrombopoietin is the growth factor that regulates platelet production. Twenty percent of platelets are stored in the spleen, and eighty percent of platelets circulate in the bloodstream.[1]
Blood Components
Red Blood Cells
Red blood cells (RBCs), also called erythrocytes, transport oxygen and carbon dioxide between tissues and the lungs. RBCs, the most abundantly formed elements in the blood, are basically sacs packed with hemoglobin that carry oxygen. For this reason, a lack of RBCs results in symptoms related to decreased oxygenation of the body’s tissues and organs. See Figure 3.1[2] for an illustration of RBCs containing hemoglobin that carries oxygen molecules.
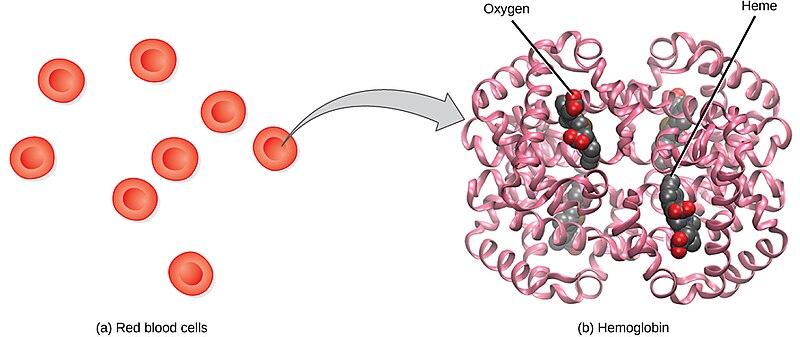
Production of RBCs in the bone marrow occurs at the staggering rate of more than 2 million cells per second. For this production to occur, raw materials, including iron, folic acid, and B12, must be present in adequate amounts.
RBCs live only 120 days on average and must be continually replaced. Old RBCs and hemoglobin are broken down by white blood cells in a process called phagocytosis. When hemoglobin breaks down, bilirubin is formed. If the body cannot adequately metabolize bilirubin, a symptomatic yellow discoloration called jaundice occurs in the skin, mucus membranes, and eyes.[3],[4],[5]
White Blood Cells
White blood cells (WBCs), also called leukocytes, provide immune function and body defenses against disease. WBCs protect the body against invading microorganisms and body cells with mutated DNA, and they also clean up debris.[6] There are five main types of WBCs called neutrophils, lymphocytes, monocytes, eosinophils, and basophils. See Figure 3.2[7] for an illustration of the different types of WBCs.
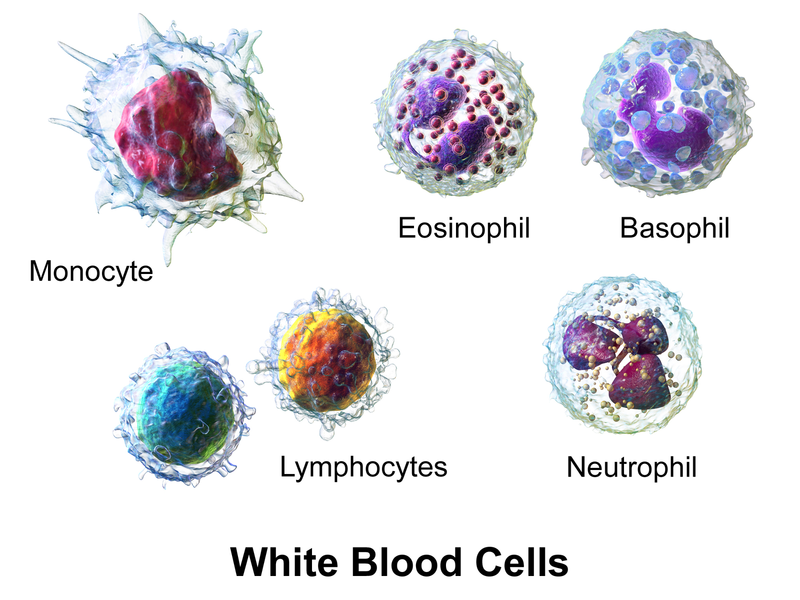
Table 3.2a summarizes the main functions of the different types of WBCs.
Table 3.2a. Types of WBCs and Functions[8],[9]
| Leukocyte Type | Percentage in Total WBC Count | Main Function |
|---|---|---|
| Neutrophils (NEUT) | 40 -70% | Phagocytosis: The engulfment and destruction of bacteria, an important process during acute bacterial infections and inflammation.
Mature neutrophils are commonly referred to as “segs,” and immature neutrophils are commonly referred to as “bands.” A “shift to the left” indicates that mature segs are being consumed at the site of an infection, and the bone marrow is rapidly creating new bands. |
| Lymphocytes (LYM) | 20-45% | Adaptive immunity: The production of antibodies called B cells and targeted killing of infected cells by T cells. |
| Monocytes (MONO) | 2-10% | Transformed into macrophages in acute infections to clear debris and pathogens via phagocytosis. Monocytes are also present in the immune response to chronic infections. |
| Eosinophils (EO) | 1-6% | Defend against parasites and modulate allergic reactions by releasing histamine and other chemicals. |
| Basophils (BASO) | 0.5-1% | Release histamine and other inflammatory mediators during an allergic reaction. |
Review information about pathogens, natural defenses against infection, inflammation, specific adaptive immunity, and infection in the “Infection” chapter of Open RN Nursing Fundamentals, 2e.[10]
Platelets
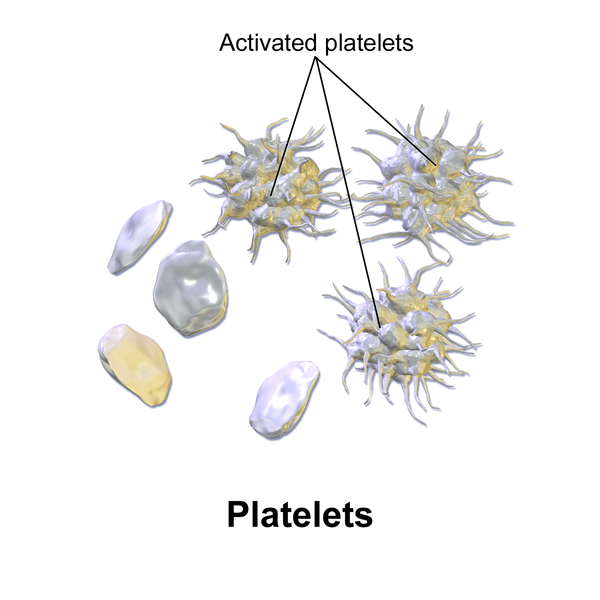
Platelets, also called thrombocytes, primarily assist in blood clotting and also release growth factors to repair and heal tissue. Platelets serve a critical function in hemostasis, the process by which the body seals a ruptured blood vessel and prevents further loss of blood. During hemostasis, platelets become activated and create a platelet plug. See Figure 3.3[11] for an illustration of platelets and activated platelets. Hemostasis and blood clot formation are further discussed in the “Blood Clot Formation” subsection.
Accessory Organs
The spleen and liver are essential accessory organs for blood production and clotting regulation.
Spleen
The spleen produces and stores WBCs, filters and stores RBCs and platelets, and plays a role in the filtration and destruction of old RBCs. There are two types of tissue in the spleen: white pulp and red pulp. White pulp, the lymphatic tissue of the spleen, produces WBCs. Red pulp serves as a storage site for RBCs and platelets. The spleen also filters antigens and plays a role in the destruction of old RBCs and the breakdown of hemoglobin.[12]
Because the spleen serves as a reservoir for blood, injury to the spleen can result in hemorrhage. For this reason, clients who have experienced blunt abdominal trauma undergo rapid diagnostic testing to check for splenic damage and hemorrhage.
Enlargement of the spleen, called splenomegaly, can occur due to a variety of reasons, such as systemic infection, sickle cell disease, venous congestion, or malignancy.
Clients who undergo splenectomy (i.e., removal of the spleen) experience reduced immune function and an increased risk of infection.[13]
Liver
The liver serves as a storage site for whole blood and blood cells. It also produces prothrombin and other blood clotting factors. Prothrombin and its role are further described in the “Blood Clot Formation” subsection.
The liver also plays a significant role in the breakdown of hemoglobin. The liver receives unconjugated bilirubin from blood circulation, where it undergoes conjugation so it can be filtered and excreted by the kidneys. If the liver is impaired and cannot adequately metabolize bilirubin, characteristic jaundice occurs.[14],[15]
Blood Clot Formation
Hemostasis is a process that leads to cessation of bleeding from a blood vessel and involves multiple interlinked steps. The steps begin when there is trauma to the lining of a blood vessel and culminates into the formation of a “plug” that closes the damaged site of the blood vessel and controls the bleeding.[16]
Hemostasis can be divided into four stages: 1) injury to the blood vessel, 2) vascular spasms of the injured blood vessel, 3) formation of a temporary “platelet plug,” and 4) coagulation and formation of the fibrin clot (i.e., the blood clot).[17] Failure of any of these stages of hemostasis can result in hemorrhage.[18] See Figure 3.4[19] for an illustration of these steps of hemostasis.
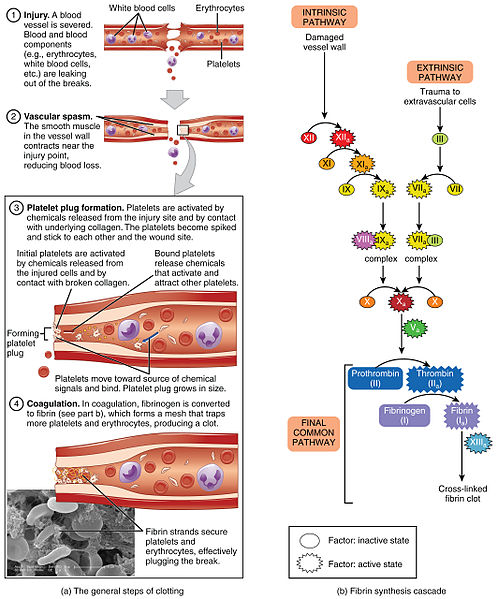
Vascular Spasm
Vascular spasm occurs after a blood vessel is injured. Vasoconstriction occurs when smooth muscle in the vessel wall contracts near the point of injury to reduce the amount of blood loss.
Platelet Plug Formation
The next step of hemostasis is creation of a platelet plug. Platelets are activated by chemicals released by the injured vessel site and by contact with the underlying collagen. The activated platelets become spiked and stick to one another at the wound site in a process called platelet aggregation.
The key steps of platelet aggregation are as follows:
- Adhesion: Platelets have receptors on their surfaces that allow them to adhere to the exposed collagen and other proteins at the site of injury.
- Activation: Upon adhesion, platelets become activated and change their shape. They release chemical signals, which attract and activate more platelets.
- Aggregation: The activated platelets stick together and form a platelet plug at the site of injury. This plug helps to temporarily seal the wound.[20],[21]
Coagulation Cascade
The final step of hemostasis is the coagulation cascade. The coagulation cascade is a complex series of reactions involving a variety of coagulation factors that eventually leads to the formation of a fibrin clot, a fibrous protein that strengthens the platelet plug and creates what is commonly called a “blood clot.”
The key steps of the coagulation cascade include the following:
- Activation of clotting factors: When tissue is injured, a series of clotting factors are activated. This involves a cascade of enzyme-mediated reactions, with each activated clotting factor activating the next one in the sequence.
- Formation of prothrombinase complex: One of the crucial steps of the coagulation cascade is the formation of prothrombinase, which converts the inactive prothrombin into its active form, thrombin. Thrombin plays a central role in the next step of hemostasis.
- Conversion of fibrinogen to fibrin: Thrombin catalyzes the conversion of soluble fibrinogen into insoluble fibrin. Fibrin forms a mesh-like structure that traps red blood cells, platelets, and other blood components to create a stable blood clot.[22],[23]
Fibrin clot formation is the final stage of the blood clotting process. Fibrin forms a network of fibers, reinforcing the initial platelet plug and stabilizing the clot.
These are the key steps of fibrin clot formation:
- Activation of fibrin: Thrombin activates fibrinogen, causing it to form long fibrin strands. These strands then intertwine to create a mesh-like network.
- Cross-linking of fibrin: Enzymes help to cross-link the fibrin strands, strengthening the clot’s structure further.
- Clot retraction: Once the fibrin clot is formed, platelets within the clot contract, pulling the fibrin strands closer together and compacting the clot. This clot retraction contributes to wound closure.
Fibrinolysis
Fibrinolysis is a natural mechanism that occurs in the body to help break down blood clots to prevent clots from becoming too large and blocking blood vessels. The process of fibrinolysis is driven by an enzyme called plasmin. Plasmin is responsible for breaking down fibrin, the mesh-like structure of a blood clot.[24],[25]
The key steps of fibrinolysis are as follows:
- Activation of plasminogen: Plasminogen is an inactive precursor of plasmin that is always present in theplasminblood. Plasminogen only becomes active when a blood clot is formed and needs to be degraded. When this occurs, substances trigger the conversion of plasminogen into its active form plasmin.
- Formation of tissue plasminogen activator (tPA): Tissue plasminogen activator is a substance released by endothelial cells lining blood vessels. Tissue plasminogen activator can also be administered as a medical intervention in occurrence of a stroke or if an individual experiences a cardiac blockage. Tissue plasminogen activator is the substance that binds to plasminogen, causing the conversion into active plasmin.
- Plasmin action: Active plasmin then begins to break the fibrin strands within the blood clot. It breaks the fibrin into smaller and smaller products.
- Dissolution of the clot: As plasmin continues to break apart the fibrin strands, the blood clot gradually dissolves. This makes the clot continue to decrease in size and restores blood flow.[26],[27]
Fibrinolysis plays an important part in hemostasis to not only ensure that blood clots form to control bleeding but also dissolve appropriately to allow blood flow through the vessel. Disorders in fibrinolysis can result in health alterations related to thrombosis (clot formation) or hemorrhage (increased risk of bleeding).[28],[29] Warfarin is a commonly prescribed medication that disrupts the coagulation cascade to prevent clot formation in clients who are at risk. Read more about warfarin in the following box.
The Role of Warfarin
Warfarin disrupts the coagulation cascade by specifically targeting vitamin K, an essential nutrient required for the synthesis of several clotting factors in the liver. Vitamin K is responsible for activating clotting factors by modifying them into their active forms.
Warfarin acts as a vitamin K antagonist, meaning it interferes with the function of vitamin K and reduces the production of active clotting factors. It does this by blocking the enzyme responsible for recycling vitamin K, known as vitamin K epoxide reductase. Without enough vitamin K available, the liver is unable to produce fully functional clotting factors, which slows down the clotting process. For this reason, clients on warfarin must eat a consistent amount of vitamin K in their diet.
This anticoagulant effect of warfarin is carefully controlled to achieve the desired therapeutic effect. The dosage of warfarin for each individual is carefully adjusted to ensure their ability to clot is neither too fast nor too slow. Too much warfarin can lead to excessive bleeding, whereas not enough warfarin can allow disruptive clot formation.
For this reason, routine monitoring of a client’s International Normalized Ratio (INR) is essential during warfarin therapy. The INR is a blood test that measures how long it takes for the blood to clot compared to a standardized value. By monitoring the INR regularly, health care providers adjust each client’s warfarin dosage as needed to maintain the appropriate anticoagulant effect.
- Leukemia Foundation. (2024). The bone marrow and blood formation. https://www.leukaemia.org.au/blood-cancer/understanding-your-blood/bone-marrow-and-blood-formation/ ↵
- “Figure_39_04_01.jpg” by CNX OpenStax is licensed under CC BY 4.0 ↵
- Ernstmeyer, K., & Christman, E. (Eds.). (2024). Medical terminology 2e. Open RN | WisTech Open. https://wtcs.pressbooks.pub/medterm/ ↵
- Kalakonda, A., Jenkins, B. A., & John, S. (2022). Physiology, Bilirubin. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK470290/ ↵
- Philips, M. M. (2023). Jaundice causes. National Institutes of Health. Medline Plus. https://medlineplus.gov/ency/article/007491.htm ↵
- Ernstmeyer, K., & Christman, E. (Eds.). (2024). Medical terminology 2e. Open RN | WisTech Open. https://wtcs.pressbooks.pub/medterm/ ↵
- “Blausen_0909_WhiteBloodCells.png” by Blausen.com staff (2014). “Medical gallery of Blausen Medical 2014” is licensed under CC BY 3.0 ↵
- Ernstmeyer, K., & Christman, E. (Eds.). (2024). Medical terminology 2e. Open RN | WisTech Open. https://wtcs.pressbooks.pub/medterm/ ↵
- American Society of Hematology. (n.d.). Blood basics. https://www.hematology.org/education/patients/blood-basics ↵
- Ernstmeyer, K., & Christman, E. (Eds.). (2024). Nursing fundamentals 2E. Open RN | WisTech Open. https://wtcs.pressbooks.pub/nursingfundamentals/ ↵
- “Blausen_0740_Platelets.png” by Blausen.com staff (2014). “Medical gallery of Blausen Medical 2014” is licensed under CC BY 3.0 ↵
- Kapila, V., Wehrle, C.J., & Tuma, F. (2023). Physiology, Spleen. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK537307/ ↵
- Kapila, V., Wehrle, C.J., & Tuma, F. (2023). Physiology, Spleen. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK537307/ ↵
- American Society of Hematology. (n.d.). Blood basics. https://www.hematology.org/education/patients/blood-basics ↵
- Kalra, A., Yetiskul, E., & Wehrle, C.J. (2023). Physiology, Liver. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK535438/ ↵
- LaPelusa, A., & Dave, H.D. (2023). Physiology, Hemostasis. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK545263/ ↵
- LaPelusa, A., & Dave, H.D. (2023). Physiology, Hemostasis. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK545263/ ↵
- American Society of Hematology. (n.d.). Blood basics. https://www.hematology.org/education/patients/blood-basics ↵
- “1909_Blood_Clotting.jpg” by OpenStax College is licensed under CC BY 3.0 ↵
- Chaudhry, R., Usama, S.M., & Babiker, H.M. (2023). Physiology, Coagulation Pathways. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK482253/ ↵
- Leung, L. (2023). Overview of hemostasis. UpToDate. https://www.uptodate.com ↵
- Chaudhry, R., Usama, S.M., & Babiker, H.M. (2023). Physiology, Coagulation Pathways. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK482253/ ↵
- Leung, L. (2023). Overview of hemostasis. UpToDate. https://www.uptodate.com ↵
- Chaudhry, R., Usama, S.M., & Babiker, H.M. (2023). Physiology, Coagulation Pathways. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK482253/ ↵
- Leung, L. (2023). Overview of hemostasis. UpToDate. https://www.uptodate.com ↵
- Chaudhry, R., Usama, S.M., & Babiker, H.M. (2023). Physiology, Coagulation Pathways. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK482253/ ↵
- Leung, L. (2023). Overview of hemostasis. UpToDate. https://www.uptodate.com ↵
- Chaudhry, R., Usama, S.M., & Babiker, H.M. (2023). Physiology, Coagulation Pathways. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK482253/ ↵
- Leung, L. (2023). Overview of hemostasis. UpToDate. https://www.uptodate.com ↵
An enzyme produced by the kidneys in response to hypoxia and essential for RBC production.
The growth factor that regulates platelet production.
Transport oxygen and carbon dioxide between tissues and the lungs; also called erythrocytes.
Red blood cells (RBCs).
A yellow discoloration in the skin, mucus membranes, and eyes due to the body’s inability to adequately metabolize bilirubin.
Provide immune function and body defenses against disease; also called leukocytes.
White blood cells (WBCs).
The engulfment and destruction of bacteria, an important process during acute bacterial infections and inflammation.
The production of antibodies called B cells and targeted killing of infected cells by T cells.
Assist in blood clotting and release growth factors to repair and heal tissue; also called thrombocytes.
Platelets.
The process by which the body seals a ruptured blood vessel and prevents further loss of blood.
Enlargement of the spleen.
Surgical removal of the spleen.
Platelets stick together.
Occurs when activated platelets stick together at the site of an injury, which helps to temporarily seal the wound.
A complex series of reactions involving a variety of coagulation factors that eventually leads to the formation of a fibrin clot.
The final step in the clotting process.
The process in which a clot is degraded in a healing vessel.
Enzyme which drives the process of fibrinolysis
A blood test that measures how long it takes for the blood to clot compared to a standardized value.

