9.4 Antidiabetics
Open Resources for Nursing (Open RN)
Pancreatic Basics: A&P Review
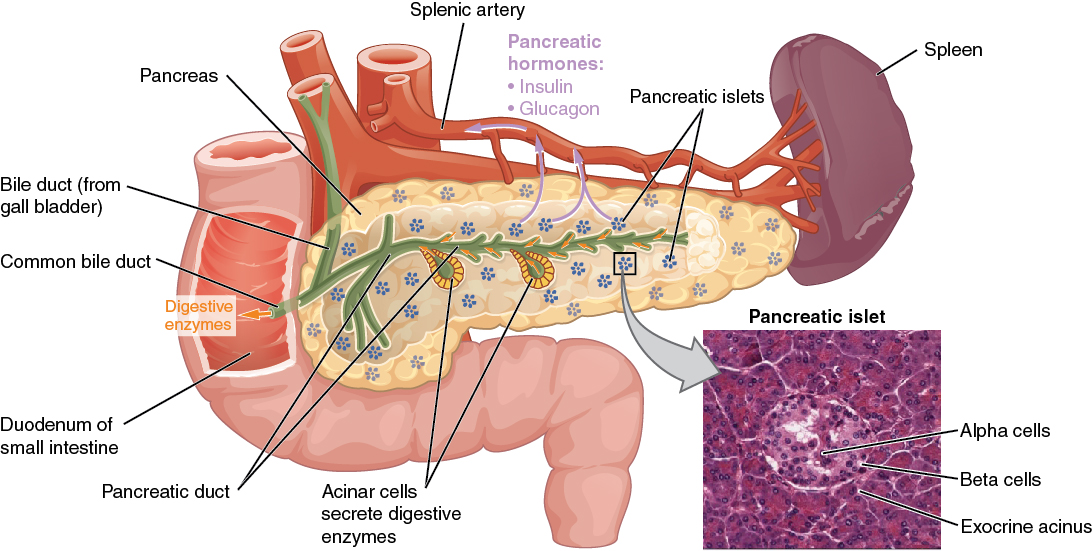
Pancreas
The pancreas is a long, slender organ located near the stomach (see Figure 9.7).[1] Although it is primarily an exocrine gland, secreting a variety of digestive enzymes, the pancreas also has an endocrine function. Pancreatic islets, clusters of cells formerly known as the islets of Langerhans, secrete glucagon and insulin. Glucagon plays an important role in blood glucose regulation because low blood glucose levels stimulate its release. On the other hand, elevated blood glucose levels stimulate the release of insulin.
Regulation of Blood Glucose Levels by Insulin and Glucagon
Glucose is the preferred fuel for all body cells. The body derives glucose from the breakdown of the carbohydrate-containing foods and drinks we consume. Glucose not immediately taken up by cells for fuel can be stored by the liver and muscles as glycogen or converted to triglycerides and stored in the adipose tissue. Hormones regulate both the storage and the utilization of glucose as required. Receptors located in the pancreas sense blood glucose levels, and subsequently, the pancreatic cells secrete glucagon or insulin to maintain normal levels.
Glucagon
Receptors in the pancreas can sense the decline in blood glucose levels, such as during periods of fasting or during prolonged labor or exercise. In response, the alpha cells of the pancreas secrete the hormone glucagon, which has several effects:
- It stimulates the liver to convert stores of glycogen back into glucose. This response is known as glycogenolysis. The glucose is then released into the circulation for use by body cells.
- It stimulates the liver to take up amino acids from the blood and convert them into glucose. This response is known as gluconeogenesis.
- It stimulates lipolysis, the breakdown of stored triglycerides into free fatty acids and glycerol. Some of the free glycerol released into the bloodstream travels to the liver, which converts it into glucose. This is also a form of gluconeogenesis.
Taken together, these actions increase blood glucose levels. The activity of glucagon is regulated through a negative feedback mechanism; rising blood glucose levels inhibit further glucagon production and secretion. (See Figure 9.8 for an illustration of homeostatic regulation of blood glucose levels.)[2]
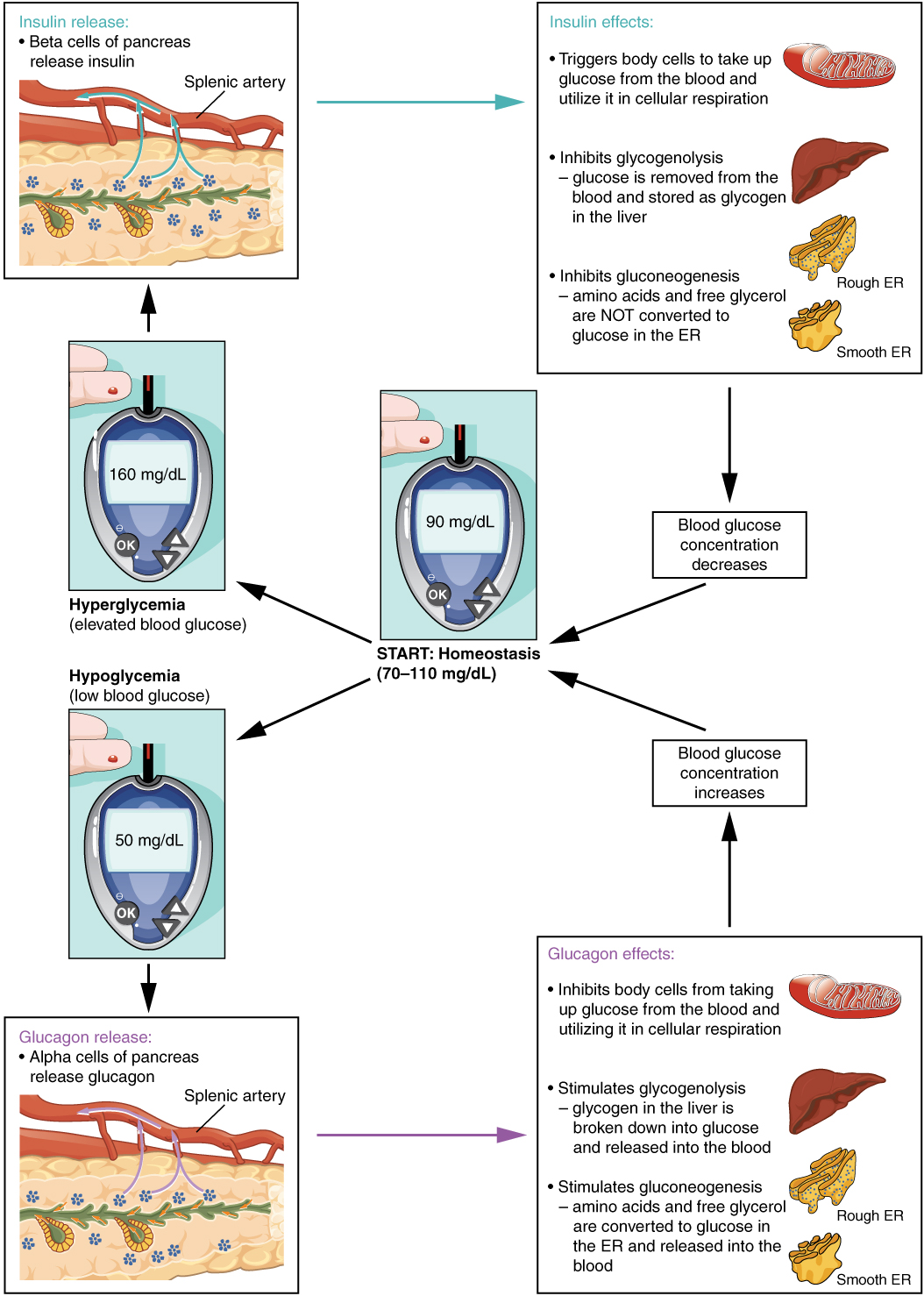
Insulin
Insulin facilitates the uptake of glucose into skeletal and adipose body cells. The presence of food in the intestine triggers the release of gastrointestinal tract hormones. This, in turn, triggers insulin production and secretion by the beta cells of the pancreas. Once nutrient absorption occurs, the resulting surge in blood glucose levels further stimulates insulin secretion.
Insulin triggers the rapid movement of glucose transporter vesicles to the cell membrane, where they are exposed to the extracellular fluid. The transporters then move glucose by facilitated diffusion into the cell interior.
Insulin also reduces blood glucose levels by stimulating glycolysis, the metabolism of glucose for generation of ATP. It further stimulates the liver to convert excess glucose into glycogen for storage, and it inhibits enzymes involved in glycogenolysis and gluconeogenesis. Finally, insulin promotes triglyceride and protein synthesis. The secretion of insulin is regulated through a negative feedback mechanism. As blood glucose levels decrease, further insulin release is inhibited.
Disorders of the Endocrine System: Diabetes Mellitus
Dysfunction of insulin production and secretion, as well as the target cells’ responsiveness to insulin, can lead to a condition called diabetes mellitus, a common disease that affects the ability of the body to produce and/or utilize insulin. There are two main forms of diabetes mellitus. Type 1 diabetes is an autoimmune disease affecting the beta cells of the pancreas. The beta cells of people with type 1 diabetes do not produce insulin; thus, synthetic insulin must be administered by injection or infusion. Type 2 diabetes accounts for approximately 95 percent of all cases. It is acquired, and lifestyle factors such as poor diet and inactivity greatly increase a person’s risk. In type 2 diabetes, the body’s cells become resistant to the effects of insulin. In response, the pancreas increases its insulin secretion, but over time, the beta cells become exhausted. In many cases, type 2 diabetes can be reversed by moderate weight loss, regular physical activity, and consumption of a healthy diet. However, if blood glucose levels cannot be controlled, oral diabetic medication is implemented and eventually the type 2 diabetic may require insulin.
Diabetes is diagnosed when lab tests reveal that blood glucose levels are higher than normal, a condition called hyperglycemia.[3] According to the American Diabetes Association (ADA), normal fasting blood glucose levels are 80-130 mg/dL. Glycosylated hemoglobin, also called A1C, is used to assess long-term blood glucose levels over 3 months. The ADA states that A1C target levels vary according to age and health, but the generalized A1C target is less than 7%.[4]
Nursing Considerations
Assessment
Diabetic patients should be continuously monitored for signs of hypoglycemia and hyperglycemia. When a diabetic patient is experiencing stress or an infection, the nurse should plan to assess the blood glucose levels more frequently.
Implementation
The nurse should follow agency policy and ISMP guidelines for safe insulin administration. See the “Legal Ethical” chapter for more information about ISMP guidelines. Onset and peak times of insulin and sulfonylureas, in association with anticipated meal times, should always be considered to avoid hypoglycemia episodes. If a hypoglycemia episode occurs, the nurse should intervene quickly using the agency’s established hypoglycemia protocol, and the event should be reported to the provider and in the shift-to-shift report. Symptomatic hyperglycemia should be immediately reported to the provider. Patient education should be provided to patients, family members, and/or caregivers according to ADA and ISMP guidelines.
Evaluation
The nurse should evaluate A1C levels to determine effectiveness (and compliance with) the treatment regimen.
Diabetic Medication Classes: Insulins
Because the hallmark of type 1 diabetes is absent or near-absent β-cell function, insulin treatment is essential for individuals with type 1 diabetes. Current evidence-based recommendations regarding pharmacological treatment of type 1 diabetes from the American Diabetes Association (ADA) include:
- Most people with type 1 diabetes should be treated with multiple daily injections of prandial and basal insulin or continuous subcutaneous insulin infusion.
- Most individuals with type 1 diabetes should use rapid-acting insulin analogs to reduce hypoglycemia risk.
- Individuals with type 1 diabetes on prandial insulin doses should be educated on carbohydrate intake, premeal blood glucose levels, and anticipated physical activity.
- Individuals with type 1 diabetes who have been successfully using continuous subcutaneous insulin infusion should have continued access to this therapy after they turn 65 years of age.
Basal insulin can be long-acting (insulin glargine or insulin detemir) or intermediate-acting (insulin isophane suspension [NPH]). Prandial insulins are used with meals and may be rapid acting (insulin lispro, insulin aspart, or insulin glulisine) or short acting (regular insulin).
According to the ADA, insulin requirements can be estimated based on weight, with typical doses ranging from 0.4 to 1.0 units/kg/day. Higher amounts are required during puberty, pregnancy, and medical illness. Physiologic insulin secretion varies with glycemia, meal size, and tissue demands for glucose. To approach this variability in people using insulin treatment, strategies have evolved to adjust meal-time doses based on predicted needs. Thus, education of patients on how to adjust insulin to account for carbohydrate intake, premeal glucose levels, and anticipated activity is important. Ensuring that patients and/or caregivers understand correct insulin injection technique is also important to optimize glucose control and insulin use safety.[5]
Patients on insulin therapy are at risk for hypoglycemia. It is essential for the nurse to monitor for signs of hypoglycemia and to intervene appropriately. See Figure 9.9 for symptoms of hypoglycemia. Hypoglycemia is defined as a blood glucose level below 70 mg/dL; severe hypoglycemia refers to a blood glucose level below 40 mg/dL.
Hypoglycemia Symptoms
| Mild-to-Moderate | Severe |
|---|---|
|
|
Figure 9.9 Hypoglycemia Symptoms
If a patient with diabetes shows a sudden change in mood or mental status or other symptoms of hypoglycemia, the nurse should immediately check the blood glucose level. Healthcare agencies use hypoglycemia protocols so that the nurse can react quickly to episodes of hypoglycemia before they become severe. Hypoglycemia protocols contain orders for immediate treatment by the nurse. For instance, in patients who can tolerate oral intake, 15 grams of rapidly digested carbohydrates (such as 4 ounces of fruit juice) are recommended. In patients who are NPO or can’t take oral treatment, dextrose 50% IV or glucagon IM or subcutaneously are administered. Patients who have had a hypoglycemic episode should be monitored closely for the following 24 hours because they are at increased risk for another episode. The provider and the oncoming nurse should be notified of hypoglycemia episodes to discuss the possible cause of the hypoglycemic event and make insulin adjustments, if needed, to avoid additional hypoglycemia. Tracking hypoglycemia episodes and analyzing causes are important performance improvement activities.[6]
The arrival of continuous glucose monitors to clinical practice has been proven to reduce nocturnal hypoglycemia in people using insulin pumps with glucose sensors due to automatic suspension of insulin delivery at a preset glucose level. The U.S. Food and Drug Administration has also approved the first hybrid closed-loop pump system.[7] A hybrid closed-loop pump system automatically adjusts basal insulin delivery every 5 minutes based on sensor glucose to maintain blood glucose levels as close to a specific target as possible.[8]
According to the ADA, lifestyle modifications that improve health should be emphasized, along with any pharmacologic therapy. Lifestyle modifications include healthy food choices to stabilize blood glucose levels, as well as daily exercise.
Hypokalemia
All insulin products cause a shift in potassium from the extracellular to intracellular space, which can possibly lead to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia due to other medications such as diuretics.
Insulin Pens
Insulin pens are often used in inpatient settings, as well as for self-administration, to facilitate safe and accurate self-administration of insulin. See Figure 9.10 for an image of an insulin pen.[9] According to the ISMP, insulin pens offer several advantages over vials beyond dosing accuracy, convenience, and ease of use:
- Each pen is already labeled by the manufacturer with the product name and product barcode (whereas syringes of insulin prepared on the patient care unit from vials run the risk of being unlabeled).
- Each pen can be individually labeled with the patient’s name (and ideally with a patient-specific barcode).
- The pen provides the patient’s insulin in a form ready for administration.
- The pen lessens nursing time needed to prepare and administer insulin.
- Insulin pens reduce medication waste that can occur when dispensing 10 mL-sized insulin vials for each patient.
However, improper sharing of insulin pens among multiple patients has exposed patients to bloodborne pathogens. Insulin pens should never be reused for multiple patients; even if the needle is changed between patients, there can still be body fluid exposure.[10]
High-Alert Medication and Prevention of Errors
Insulin is a high-alert medication that can be associated with significant patient harm when used in error. A variety of error types have been associated with insulin therapy, including administration of the wrong insulin product, improper dosing (underdosing and overdosing), dose omissions, incorrect use of insulin delivery devices, wrong route (intramuscular versus subcutaneous), and improper patient monitoring. Many errors result in serious hypoglycemia or hyperglycemia. Hypoglycemia is often caused by a failure to adjust insulin therapy in response to a reduction in nutritional intake or an excessive insulin dose stemming from a prescribing or dose measurement error. Other factors that contribute to serious hypoglycemia include inappropriate timing of insulin doses with food intake, creatinine clearance, body weight, changes in medications that affect blood glucose levels, poor communication during patient transfer to different care teams, and poor coordination of blood glucose testing with insulin administration at meal time.
In an inpatient setting, manifestations of poor glycemic control, including severe hypoglycemia and hyperglycemia, are deemed hospital-acquired conditions by the Centers for Medicare and Medicaid Services (CMS). CMS notes that poor glycemic control can be reasonably prevented with implementation of evidence-based guidelines; thus, the CMS denies payment for diabetic ketoacidosis, hypoglycemic coma, and other serious conditions related to poor glycemic control. Agency policies and procedures should be closely followed to avoid these errors and manifestations of poor glycemic control.
One strategy for look-alike medications such as Humalog and Humalin is tall man lettering on the label. Tall man lettering describes a method for differentiating the unique letter characters of similar drug names known to be confused with one another, such as HumaLOG and HumaLIN.
ISMP recommends the following safe practice guidelines for the administration of insulin by the nurse:
- Patient-specific insulin pens are stored on clinical units in a manner that prevents their inadvertent use on more than one patient.
- A coordinated process is developed to ensure timely blood glucose checks and administration of prandial insulin in conjunction with meal delivery.
- Verbal communication of point-of-care blood glucose value results are avoided as much as possible and are NEVER routinely used as the only source of information when determining insulin doses.
- Appropriately label all clinician-prepared syringes of subcutaneous insulin, unless the medication is prepared at the patient’s bedside and is immediately administered to the patient without any break in the process.
- Prior to subcutaneous insulin administration, the practitioner:
- Confirms that there is an appropriate indication
- Assesses the patient’s most current blood glucose value
- Assesses the patient for symptoms of hypoglycemia
- Informs the patient of their most current blood glucose level
- Informs the patient of their dose, the full name of the product, and the insulin’s intended action
- An individual insulin pen is never used for more than one patient.
- Barcode scanning is used to verify that a patient-specific pen is used to administer the correct insulin to the correct patient.
- Prior to transitions of care, a process is in place to ensure that patients will have the necessary prescriptions, supplies, a follow-up care plan, and printed instructions for all prescribed insulin and blood glucose monitoring.
- Patients discharged on insulin are assessed for understanding of their self-management, including:
- Demonstration of proper dose measurement and self-administration using the same administration device that will be used at home (e.g., vial and syringe, pen, pump)
- Correct monitoring of blood glucose values
- The signs and symptoms of hyper- and hypoglycemia and how to respond if these symptoms occur
- Common types of errors possible with their insulin therapy and how to prevent or detect these errors
- The importance of regular follow-up with their primary care provider/specialist, including the date of their next appointment
- Patients who self-administer concentrated U-500 insulin using a vial and syringe are taught to use only a U-500 syringe and communicate their doses in terms of the name and concentration of the insulin and the actual dose in units using only the U-500 syringe[11]
Lifespan Considerations
Elderly
The elderly are at higher risk for hypoglycemia episodes. The ADA has the following recommendations for elderly patients with diabetes:
- In older adults at increased risk of hypoglycemia, medication classes with low risk of hypoglycemia are preferred.
- Overtreatment of diabetes is common in older adults and should be avoided.
- Deintensification (or simplification) of complex regimens is recommended to reduce the risk of hypoglycemia, if it can be achieved within the individualized A1C target.[12]
Children and Adolescents
Type 1 diabetes is the most common form of diabetes in youth. Unique aspects of care and management of children and adolescents with type 1 diabetes must be considered, such as changes in insulin sensitivity related to physical growth and sexual maturation, ability to provide self-care, supervision in the child care and school environment, neurological vulnerability to hypoglycemia and hyperglycemia in young children, as well as possible adverse neurocognitive effects of diabetic ketoacidosis (DKA). Evidence-based ADA recommendations for glycemic control for children and adolescents include:
- The majority of children and adolescents with type 1 diabetes should be treated with intensive insulin regimens, either via multiple daily injections or continuous subcutaneous insulin infusion.
- All children and adolescents with type 1 diabetes should self-monitor glucose levels multiple times daily (up to 6–10 times/day), including pre-meal, pre-bedtime, and as needed for safety in specific situations such as exercise, driving, or the presence of symptoms of hypoglycemia.
- Continuous glucose monitoring should be considered in all children and adolescents with type 1 diabetes, whether using injections or continuous subcutaneous insulin infusion, as an additional tool to help improve glucose control. Benefits of continuous glucose monitoring correlate with adherence to ongoing use of the device.
- Automated insulin delivery systems appear to improve glycemic control and reduce hypoglycemia in children and should be considered in children with type 1 diabetes.
- An A1C target of <7.5% should be considered in children and adolescents with type 1 diabetes but should be individualized based on the needs and situation of the patient and family. [13]
There are several different types of insulins that vary in terms of onset, peak, and duration. It is critical for the nurse to be knowledgeable of these differences to help prevent episodes of hypoglycemia due to mismatched administration of insulin with food intake.
Rapid-Acting Insulin
Rapid-acting insulins include insulin lispro (Humalog) and insulin aspart (Novolog) and are also available via inhalation (Afrezza). See Figure 9.11 for an image of Novolog insulin.[14]
Indications
Rapid-acting insulins are also called prandial insulins because they are administered with meals to mimic the effects of endogenous insulin release when food is eaten. Dosages of rapid-acting insulin are individualized based on carbohydrate intake, premeal glucose levels, and anticipated activity.
Mechanism of Action
Insulins lower blood glucose by stimulating peripheral glucose uptake by skeletal muscle and fat and by inhibiting hepatic glucose production.
Specific Administration Considerations
Humalog-100 (100 units per ml) and Humalog-200 (200 units per ml) are administered subcutaneously; however, only Humalog-100 is administered via continuous subcutaneous injection or intravenously. Humalog-100 can only be mixed with NPH insulin, but Humalog-200 should not be mixed with other insulin. Inspect insulin visually before use. It should appear clear and colorless; do not use if particulate matter or coloration is seen. Humalog-100 is available in vials, KwikPens, and cartridges; Humalog-200 is only available in KwikPens. Administer subcutaneously into the outer lateral aspect of the upper arm, the abdomen (from below the costal margin to the iliac crest and more than two inches from the umbilicus), the anterior upper thighs, or the buttocks. Rotate injection sites within the same region from one injection to the next to reduce the risk of lipodystrophy. Lipodystrophy can be a lump or small dent in the skin that forms when a person performs injections repeatedly in the same spot.
Because of the rapid onset of insulin lispro and insulin aspart and the potential for hypoglycemia, these insulins should be administered within 15 minutes before or right after eating a meal. Peak serum levels are seen 30 to 90 minutes after dosing. Inhaled insulin enters the bloodstream within 1 minute and peaks in 30-60 minutes. Inhaled insulin is contraindicated in patients with chronic lung disease such as asthma or COPD.
Adverse effects of all insulins include hypoglycemia and hypokalemia. Inhaled insulin has a Black Box Warning for potentially causing acute bronchoconstriction.
Patient Teaching & Education
See ISMP guidelines for patient teaching in the previous section titled “High Risk Medications and Prevention of Errors.”
Short-Acting Insulin
Short-acting insulins include regular insulin with a brand name of Humulin R or Novolin R. A concentrated formulation of Humulin R u-500 is also available. See Figure 9.12 for an image of Humulin R insulin.[15]
Indications
Short-acting insulins are given with meals to mimic the effects of endogenous insulin release when food is eaten. Dosages of short-acting insulin are individualized based on carbohydrate intake, premeal glucose levels, and anticipated activity levels.
Mechanism of Action
The primary activity of insulin is the regulation of glucose metabolism. Insulin lowers blood glucose by stimulating peripheral glucose uptake, especially by skeletal muscle and fat, and by inhibiting hepatic glucose production.
Specific Administration Considerations
Regular insulin is generally administered subcutaneously. It is the only insulin that can be administered intravenously under close supervision of blood glucose and potassium levels. It is available in vials and insulin pens. Inspect insulin visually before use. It should appear clear and colorless; do not use if particulate matter or coloration is seen. Administer subcutaneously into the outer lateral aspect of the upper arm, the abdomen (from below the costal margin to the iliac crest and more than two inches from the umbilicus), the anterior upper thighs, or the buttocks. Rotate injection sites within the same region from one injection to the next to reduce the risk of lipodystrophy. Subcutaneous doses should be administered approximately 30 minutes before meals because this is the typical onset of action. Peak effects occur in 3 hours with a duration of 8 hours. Do not mix with insulin preparations other than NPH.
Humulin R u-500 should only be administered in u-500 insulin syringes to avoid dosage calculation errors.
Adverse effects of insulin include hypoglycemia and hypokalemia.
Patient Teaching & Education
See IMSP guidelines for patient teaching in the previous section entitled “High Risk Medications and Prevention of Errors.”
Intermediate-Acting Insulin
NPH insulin, also known as isophane insulin, is an intermediate–acting insulin. Brand names include Humulin-N or Novolin-N. Mixtures of short- and intermediate-acting insulin include Humulin 70/30 or Novolin 70/30.
Indications
Intermediate insulins are administered once or twice daily to mimic endogenous basal insulin levels.
Mechanism of Action
Insulins lower blood glucose by stimulating peripheral glucose uptake by skeletal muscle and fat and by inhibiting hepatic glucose production.
Specific Administration Considerations
NPH insulin is a white and cloudy suspension. Gently roll or invert vial/pen several times to re-suspend the insulin before administration. It should only be administered subcutaneously. It may be mixed with rapid-acting or short-acting insulins, but those insulins should be drawn into the syringe before the NPH is added. Administer subcutaneously into the outer lateral aspect of the upper arm, the abdomen (from below the costal margin to the iliac crest and more than two inches from the umbilicus), the anterior upper thighs, or the buttocks. Rotate injection sites within the same region from one injection to the next to reduce the risk of lipodystrophy. The onset of action and peak are affected by the site of injection, physical activity level, and other variables but the median peak level occurs in 4 hours. See Figure 9.13 for an image of Novolin-N (a cloudy insulin) that can be mixed with Novolin R (a clear insulin).[16]
Mixed medications such as Humulin 70/30 should be administered subcutaneously approximately 30 minutes before a meal. They are typically dosed twice daily (with each dose intended to cover 2 meals or a meal and a snack).
Unopened vials should be stored in the refrigerator until the expiration date. Opened vials should be labelled with the open date and stored in the refrigerator for up to 28-42 days (depending on the formulation/insulin type) and then discarded. Unopened pens should be stored in the refrigerator until the expiration date. Used pens should be stored at room temperature, but kept away from heat and light, for up to 10-28 days (depending on the formulation/insulin type) and then discarded.
Patient Teaching & Education
See IMSP guidelines for patient teaching in the previous section titled “High Risk Medications and Prevention of Errors.”
Long-Acting Insulin
Insulin glargine (Lantus) and insulin devemir (Levemir) are long-acting insulins given once or twice daily. See Figure 9.14 for an image of a levemir insulin pen.[17]

Indications
Long-acting insulins are given once or twice daily. In type 1 diabetics, long-acting insulin should be used concomitantly with rapid- or short-acting insulin at mealtimes.
Mechanism of Action
Insulins lower blood glucose by stimulating peripheral glucose uptake by skeletal muscle and fat and by inhibiting hepatic glucose production.
Specific Administration Considerations
Long-acting insulin has a relatively constant concentration/time profile over 24 hours with no pronounced peak in comparison to NPH insulin. It should only be administered subcutaneously and is available in vials and insulin pens . Inspect insulin visually before use. It should appear clear and colorless; do not use if particulate matter or coloration is seen. Administer subcutaneously into the outer lateral aspect of the upper arm, the abdomen (from below the costal margin to the iliac crest and more than two inches from the umbilicus), the anterior upper thighs, or the buttocks. Rotate injection sites within the same region from one injection to the next to reduce the risk of lipodystrophy.
Patient Teaching & Education
See ISMP guidelines for patient teaching in the previous section titled “High Risk Medications and Prevention of Errors.”
Glucagon
Indications
Glucagon is indicated as a treatment for severe hypoglycemia (low blood sugar), which may occur in patients with diabetes mellitus. Glucagon injection is used for patients who are unable to safely swallow carbohydrates to treat hypoglycemia due to the effects of hypoglycemia or other medical conditions.
Mechanism of Action
Glucagon increases blood glucose concentration during an episode of hypoglycemia. See Figure 9.15 for an image of an emergency glucagon kit.[18]
Specific Administration Considerations
Glucagon may be administered subcutaneously, intramuscularly, or intravenously. Peak glucose levels occur within 13-20 minutes of subcutaneous or IM injection.
Patient Teaching & Education
Patients with type 1 diabetes may have less of an increase in blood glucose levels compared with a stable type 2 patient, so a supplementary carbohydrate should be given as soon as possible, especially to a pediatric patient. [19]
Now let’s take a closer look at the medication grid comparing insulins in Table 9.4a.[20]
Table 9.4a Insulins Medication Grid
| Class/Subclass | Prototypes/Generics | Onset/Peak/Duration | Administration Considerations | Therapeutic Effects | Adverse/Side Effects |
|---|---|---|---|---|---|
| Rapid-Acting Insulin | insulin lispro (Humalog)
insulin aspart (Novolog) inhaled insulin (Afreeza)
|
Onset: 15-30 minutes
Peak effect: 1-3 hours Duration: 3 – 5 hours |
Administer within 15 minutes before a meal or immediately after a meal
Afrezza is contraindicated in patients with asthma or COPD |
Maintain serum blood glucose in normal range and achieve individualized target level of A1C (often 7%) | Hypoglycemia
Hypokalemia Afrezza can cause acute bronchospasm |
| Short-Acting Insulin | Humulin R
Novolin R |
Onset: 30 minutes
Peak effect: 3 hours Duration: 8 hours |
Administer 30 minutes before a meal | Maintain serum blood glucose in normal range and achieve individualized target level of A1C (often 7%) | Hypoglycemia
Hypokalemia |
| Intermediate-Acting Insulin | Humulin N
Novolin N |
Onset: 1-2 hours
Peak effect: 6 hours (range 2.8-13 hours) Duration: up to 24 hours |
Administer once or twice daily
Only administer subcutaneously Gently roll or invert vial/pen several times to re-suspend the insulin before administration |
Maintain serum blood glucose in normal range and achieve individualized target level of A1C (often 7%) | Hypoglycemia
Hypokalemia |
| Combination: Intermediate-Acting/Rapid-Acting | Humalog Mix 50/50
Humalog Mix 75/25 Novolog Mix 70/30 *First number is % intermediate-acting insulin, second number is % rapid-acting |
Onset: 15-30 minutes
Peak effect: 50/50: 1-5 hours Duration: 11-22 hours |
Administer twice daily, 15 minutes before a meal or immediately after a meal
Only administer subcutaneously Gently roll or invert vial/pen several times to re-suspend the insulin before administration |
Maintain serum blood glucose in normal range and achieve individualized target level of A1C (often 7%) | Hypoglycemia
Hypokalemia |
| Combination: Intermediate-Acting/Short-Acting | Humulin 70/30
Novolin 70/30 |
Onset: 30-90 minutes
Peak effect: 1.5-6.5 hours Duration: 18-24 hours |
Administer twice daily, 30-45 minutes before a meal
Only administer subcutaneously Gently roll or invert vial/pen several times to re-suspend the insulin before administration Do not mix with other insulin
|
Maintain serum blood glucose in normal range and achieve individualized target level of A1C (often 7%) | Hypoglycemia
Hypokalemia |
| Long-Acting Insulin | insulin glargine (Lantus)
insulin detemir (Levemir) |
Onset: 3-4 hours
Peak effect: none Duration: >24 hours |
Administer once daily (sometimes dose is split and administered twice daily)
Only administer subcutaneously Do not mix with other insulin |
Maintain serum blood glucose in normal range and achieve individualized target level of A1C (often 7%) | Hypoglycemia
Hypokalemia |
| Hyperglycemic | glucagon | May be administered subcutaneously, IM, or IV
Supplementary carbohydrate should be given as soon as possible, especially to a pediatric patient Used to reverse hypoglycemic episode if NPO administration is not appropriate |
Used to reverse hypoglycemic episode if NPO administration is not appropriate | Hyperglycemia |
General administration considerations:
- Review orders closely because they may include a standard meal dose, a “sliding scale” dose, and a carb-related dose.
- Always read drug labelling closely as there are several types of dosages and formulations.
- See agency policies and ISMP guidelines for safe administration of insulin.
- When administering with an insulin pen, after inserting the pen count to 5 before removing the needle.
General therapeutic effects:
- Maintain serum blood glucose in normal range and achieve individualized target level of A1C (often 7%)
General side effects:
- Hypoglycemia and hypokalemia
Diabetic Medication: Oral Antihyperglycemics
There are several different classes of oral antihyperglycemic drugs used in conjunction with a healthy diet and exercise for the management of type 2 diabetes. According to the American Diabetes Association, metformin is the preferred initial pharmacologic agent for the treatment of type 2 diabetes.[21]Three of the most commonly used antihyperglycemic classes and prototypes are sulfonylureas (glipizide), biguanide (metformin), and DPP-IV (sitagliptin). The mechanism of action and administration considerations for each of these prototypes are described below.
Glipizide
Mechanism of Action
Glipizide is in the sulfonylurea class of antihyperglycemic medication. The mechanism of action is the stimulation of insulin secretion from the beta cells of pancreatic islet tissue and is thus dependent on functioning beta cells in the pancreatic islets. Peak plasma concentrations occur 1 to 3 hours after a single oral dose.
Specific Administration Considerations
All sulfonylurea drugs are capable of producing severe hypoglycemia. Hypoglycemia may be difficult to recognize in the elderly and in people who are taking beta-adrenergic blocking drugs. Sulfonylurea medications should be given 30 minutes before a meal due to hypoglycemic effects.
Glipizide is contraindicated in type 1 diabetics or for use of diabetic ketoacidosis; insulin should be used to treat this condition. Treatment of patients with glucose 6-phosphate dehydrogenase (G6PD) deficiency with sulfonylurea agents can lead to hemolytic anemia.
The hypoglycemic action of sulfonylureas may be potentiated by certain drugs such as nonsteroidal anti-inflammatory agents and other drugs that are highly protein bound.
Patient Teaching & Education
Patients should take the medication at the same time each day. It is important that patients understand that the medication helps control episodes of hyperglycemia but does not cure diabetes. Patients should be instructed regarding the signs of hyperglycemia and hypoglycemia. The use of sulfonylureas and alcohol may cause a disulfiram-like reaction.
Metformin
Mechanism of Action
Metformin is in the biguanide class of antihyperglycemics. It decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization. Unlike sulfonylureas, metformin does not produce hypoglycemia. See Figure 9.16 for an image of a metformin tablet.[22]
Specific Administration Considerations
Metformin hydrochloride should be given in divided doses with meals. The therapeutic goal should be to decrease both fasting plasma glucose and glycosylated hemoglobin levels to near normal by using the lowest effective dose of metformin, either when used as monotherapy or in combination with sulfonylurea or insulin.
Common adverse reactions include diarrhea, nausea/vomiting, weakness, flatulence, indigestion, abdominal discomfort, and headache.
Metformin is contraindicated in patients with kidney disease (e.g., serum creatinine levels ≥1.5 mg/dL [males] or ≥1.4 mg/dL [females]) and should be temporarily discontinued in patients undergoing radiologic studies involving intravascular administration of iodinated contrast materials because use of such products may result in acute alteration of renal function. It is also contraindicated in patients with metabolic acidosis.
Lactic acidosis is a rare, but serious, metabolic complication that can occur due to metformin accumulation during treatment with metformin; when it occurs, it is fatal in approximately 50% of cases. The risk of lactic acidosis increases with the degree of renal dysfunction and the patient’s age. Metformin should be promptly withheld in the presence of any condition associated with hypoxemia, dehydration, or sepsis. Because impaired hepatic function may significantly limit the ability to clear lactate, metformin should be avoided in patients with hepatic disease. The onset of lactic acidosis often is subtle and accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, increasing somnolence, and nonspecific abdominal distress.
Patient Teaching & Education
Patients should take the medication at the same time each day. It is important that patients understand that the medication helps control episodes of hyperglycemia but does not cure diabetes. Patients should be instructed regarding the signs of hyperglycemia and hypoglycemia. The patient may be at risk for lactic acidosis and should report chills, low blood pressure, muscle pain, or dyspnea immediately to the healthcare provider. The use of medications like metformin can cause a metallic taste in the mouth.
Sitagliptin
Mechanism of Action
Sitagliptin is an orally-active inhibitor of dipeptidyl peptidase-4 (DPP-4) enzyme that slows the inactivation of incretin hormones involved in the regulation of glucose homeostasis and thus, increases insulin release and decreases glucagon levels in the circulation. See Figure 9.17 for an image of sitagliptin.[23]
Specific Administration Considerations
Sitagliptin is taken once daily and can be taken with or without food. It can cause hypoglycemia. Dose adjustment should occur for patients with kidney disease depending on their glomerular filtration rate. Report hypersensitivity reactions, blisters/erosions, headache, or symptoms of pancreatitis, heart failure, severe arthralgia, and upper respiratory infection.
Patient Teaching & Education
Patients should take the medication at the same time each day. It is important that patients understand that the medication helps control episodes of hyperglycemia but does not cure diabetes. Patients should be instructed regarding the signs of hyperglycemia and hypoglycemia. Patients should stop taking the medication if symptoms of hypersensitivity occur and follow up immediately with their provider to determine the next course of treatment.
Now let’s take a closer look at the medication grid comparing oral antihyperglycemics in Table 9.4b. [24]
Table 9.4b Medication Grid Comparing Oral Antihyperglycemics
| Class | Prototype | Administration Considerations | Therapeutic Effects | Adverse/Side Effects |
|---|---|---|---|---|
| Sulfonylureas | glipizide | Time with meals; peak plasma concentrations occur 1 to 3 hours after administration | Reduce fasting blood sugar and glycosylated hemoglobin to near normal | Hypoglycemia; may be potentiated by nonsteroidal anti-inflammatory agents and other drugs that are highly protein bound |
| Biguanide | metformin | Contraindicated in renal and hepatic disease
Should be temporarily discontinued in patients undergoing radiologic studies involving intravascular administration of iodinated contrast materials |
Reduce fasting blood sugar and glycosylated hemoglobin to near normal | Stop immediately if signs of lactic acidosis or any condition associated with hypoxemia, dehydration, or sepsis occurs
Common adverse effects: diarrhea, nausea/vomiting, weakness, flatulence, indigestion, abdominal discomfort, and headache |
| DPP-IV inhibitor | sitagliptin | Can be given with or without food | Reduce fasting blood sugar and glycosylated hemoglobin to near norm | Hypoglycemia
Report hypersensitivity reactions, blisters/erosions, headache, or symptoms of pancreatitis, heart failure, severe arthralgia, or upper respiratory infection |
Critical Thinking Activity 9.4

A patient with diabetes mellitus type 2 is admitted to the hospital for hip replacement surgery. The nurse reviews the following orders:
Diabetic diet with carb counting
Bedside blood glucose testing before meals and at bedtime with sliding scale Humalog insulin
Sliding scale Humalog insulin based on preprandial glucose level:
- 0-150: No coverage
- 151-175: 2 units
- 176-200: 4 units
- 201-225: 6 units
- 226-250: 8 units
- Over 250: call the provider
Insulin coverage per carbohydrate intake at meals: Humalog 2 units/carb
Metformin 1000 mg twice daily
Humulin-N 20 units at breakfast and at bedtime
Hypoglycemia protocol
- Explain the difference between type 1 and type 2 diabetes.
- The patient states that he usually does not take insulin at home. What is the likely rationale for insulin therapy while hospitalized?
- The patient’s blood sugar before breakfast is 223 and he eats 3 carbs at breakfast. What types and amounts of insulin will the nurse administer?
- The nurse reviews the patient’s morning lab results and finds a creatinine of 1.8. She plans to call the provider to discuss the impact of the results on the medications ordered. Which medication may require a dosage adjustment based on these results?
- When the nurse enters the room around 4 p.m., she discovers that the patient has become irritable and is shaky. The nurse performs a bedside blood glucose and obtains a value of 60. What is the nurse’s best response?
- What is the likely cause of the patient’s condition? Explain using the onset and peak actions of the insulin orders.
- On admission, the patient’s A1C level was 10%. What does this lab value indicate?
- The provider states the discharge plan is to initiate Lantus insulin therapy at home, based on the admitting A1C level. What patient teaching should the nurse plan to provide before discharge?
Note: Answers to the Critical Thinking activities can be found in the “Answer Key” sections at the end of the book.
- "1820 The Pancreas.jpg"" by OpenStax is licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/17-9-the-endocrine-pancreas ↵
- "1822 The Homostatic Regulation of Blood Glucose Levels.jpg" by OpenStax is licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/17-9-the-endocrine-pancreas ↵
- This work is a derivative of Anatomy and Physiology by OpenStax licensed under CC BY 4.0. Access for free at https://openstax.org/books/anatomy-and-physiology/pages/1-introduction ↵
- American Diabetes Association. (2019). The Big Picture: Checking your Blood Glucose. https://www.diabetes.org/diabetes/medication-management/blood-glucose-testing-and-control/checking-your-blood-glucose ↵
- American Diabetes Association. (2019). 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care 42(S1). https://doi.org/10.2337/dc19-S009 ↵
- Seggelke, S., Everhart, B. (2012, September 11) Managing glucose levels in hospital patients. American Nurse Today. https://www.americannursetoday.com/managing-glucose-levels-in-hospital-patients/. ↵
- American Diabetes Association. (2019). 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care 42(S1). https://doi.org/10.2337/dc19-S009 ↵
- Weaver, H., Hirsch, I (2018, June 6). The Hybrid Closed Loop System: Evolution and Practical Applications. Diabetes Technology & Therapeutics 20(S2). https://www.liebertpub.com/doi/10.1089/dia.2018.0091. ↵
- "Human insulin 100 IU-1ml pen yellow background (02).jpg" by Wesalius is licensed under CC BY 4.0 ↵
- Institute for Safe Medication Practices. (2017). ISMP Guidelines for Optimizing Safe Subcutaneous Insulin Use in Adults. https://www.ismp.org/sites/default/files/attachments/2017-11/ISMP138-Insulin%20Guideline-051517-2-WEB.pdf ↵
- Institute for Safe Medication Practices. (2017). ISMP Guidelines for Optimizing Safe Subcutaneous Insulin Use in Adults. https://www.ismp.org/sites/default/files/attachments/2017-11/ISMP138-Insulin%20Guideline-051517-2-WEB.pdf ↵
- American Diabetes Association (2019) 12. Older adults: Standards of Medical Care in Diabetes—2019. Diabetes Care 42(S1). https://doi.org/10.2337/dc19-S012 ↵
- American Diabetes Association. (2019). 13. Children and adolescents: Standards of Medical Care in Diabetes—2019. Diabetes Care 42(S1). https://doi.org/10.2337/dc19-S013 ↵
- "Untitled" from Pxhere website is licensed under CC0 ↵
- "Humulin R, Insulin, 1987" by National Museum of American History Smithsonian Institution is licensed under CC BY-NC-ND 2.0 ↵
- "The Gift of Life" by Melissa Johnson is licensed under CC BY 2.0 ↵
- "Insulin analog 100 IU-1ml penfill levemir yellow background.jpg" by Wesalius is licensed under CC BY 4.0 ↵
- "Glucagon emergency rescue kit.JPG" by mbbradford is licensed under CC0 ↵
- This work is a derivative of Daily Med by U.S. National Library of Medicine in the public domain. ↵
- This work is a derivative of Daily Med by U.S. National Library of Medicine in the public domain. ↵
- American Diabetes Association. (2019). 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care 42(S1). https://doi.org/10.2337/dc19-S009 ↵
- "Metformin 500mg Tablets.jpg" by User:Ash is licensed under CC0 ↵
- "Januvia sitagliptin.jpg" by Kimivanil is licensed under CC BY-SA 4.0 ↵
- This work is a derivative of Daily Med by U.S. National Library of Medicine in the public domain. ↵
Gland that secretes digestive enzymes.
A hormone that facilitates the uptake of glucose into skeletal and adipose body cells.
Stimulated by insulin, the metabolism of glucose for generation of ATP.
An autoimmune disease that affects the beta cells of the pancreas so they do not produce insulin; thus, synthetic insulin must be administered by injection or infusion.
A condition where the body’s cells become resistant to the effects of insulin. Over time, the beta cells become exhausted and if blood glucose levels cannot be controlled through a healthy diet and exercise, then oral diabetic medication must be implemented and eventually insulin administration may be required.
Elevated blood sugar.
Long-acting (insulin glargine or insulin detemir) or intermediate-acting (NPH) insulin.
During or relating to the eating of food.
A blood glucose level below 70 mg/dL; severe hypoglycemia refers to a blood glucose level below 40.

