10.7 Opioid Analgesics and Antagonists
Open Resources for Nursing (Open RN)
Opioid analgesics are prescribed for moderate and severe pain. See Figure 10.8 in the “Nursing Process” section for a list of common opioid medications used to treat moderation pain to severe pain. As discussed in that section, morphine is at the top of the WHO ladder and is used to treat severe pain. It is also commonly used to treat cancer pain and for pain at end of life because there is no “ceiling effect,” meaning the higher the dose, the higher the level of analgesia. Morphine is also commonly used in patient controlled analgesia (PCA); other medications administered via PCA include hydromorphone or fentanyl. To receive the opioid using a PCA device, the patient pushes a button, which releases a specific dose but also has a lockout mechanism to prevent an overdose.[1]
Morphine
Morphine is an example of an opioid used to treat moderate to severe pain.
Mechanism of Action
Morphine binds to opioid receptors in the CNS and alters the perception of and response to painful stimuli while producing generalized CNS depression.
Indications for Use
Morphine is indicated for the relief of moderate to severe acute and chronic pain and for pulmonary edema.
Nursing Considerations Across the Lifespan
Morphine is safe for all ages. Use cautiously with patients with liver and renal impairment.
Adverse/Side Effects
Adverse effects include respiratory depression, hypotension, light-headedness, dizziness, sedation, constipation, nausea, vomiting, and sweating.
Patient Teaching & Education
Patients should be advised regarding the risks associated with opioid analgesic use. Please see the outlined “Special Considerations” for usage below.[2]
Black Box Warning
The risk of serious adverse reactions, including slowed or difficulty breathing and death, have been reported with the combined effects of morphine with other CNS depressants. Naloxone is used to reverse opioid overdose. There is also a risk of drug abuse and dependence with morphine.
Special Considerations
Respiratory Depression
Respiratory depression is the primary risk of morphine sulfate. Respiratory depression occurs more frequently in elderly or debilitated patients and in those suffering from conditions accompanied by hypoxia, hypercapnia, or upper airway obstruction, for whom even moderate therapeutic doses may significantly decrease pulmonary ventilation.
Use morphine with extreme caution in patients with chronic obstructive pulmonary disease or cor pulmonale and in patients having a substantially decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression. In such patients, even usual therapeutic doses of morphine sulfate may increase airway resistance and decrease respiratory drive to the point of apnea. Consider alternative non-opioid analgesics, and use morphine sulfate only under careful medical supervision at the lowest effective dose in such patients.
Misuse, Abuse, and Diversion of Opioids
Morphine sulfate is an opioid agonist and a Schedule II controlled substance. Such drugs are sought by drug abusers and people with addiction disorders. Diversion of Schedule II products is an act subject to criminal penalty.
Morphine can be abused in a manner similar to other opioid agonists, legal or illicit. This should be considered when prescribing or dispensing morphine sulfate in situations where there is increased risk of misuse, abuse, or diversion. Morphine may be abused by crushing, chewing, snorting, or injecting the product. These practices pose a significant risk to the abuser that could result in overdose and death.
Interactions with Alcohol and Drugs of Abuse
Morphine has addictive effects when used in conjunction with alcohol, other opioids, or illicit drugs that cause central nervous system depression because respiratory depression, hypotension, profound sedation, coma, or death may result.
Use In Head Injury and Increased Intracranial Pressure
In the presence of head injury, intracranial lesions, or a preexisting increase in intracranial pressure, the possible respiratory depressant effects of morphine and its potential to elevate cerebrospinal fluid pressure may be markedly exaggerated. Furthermore, morphine can produce effects on pupillary response and consciousness, which may obscure neurologic signs of increased intracranial pressure in patients with head injuries.
Hypotensive Effect
Morphine may cause severe hypotension in individuals unable to maintain blood pressure who have already been compromised by a depleted blood volume or drug administration of phenothiazines or general anesthetics.
Administer morphine sulfate with caution to patients in circulatory shock, as vasodilation produced by the drug may further reduce cardiac output and blood pressure.
Gastrointestinal Effects
Do not administer morphine to patients with gastrointestinal obstruction, especially paralytic ileus because morphine diminishes propulsive peristaltic waves in the gastrointestinal tract and may prolong the obstruction.
The administration of morphine sulfate may obscure the diagnosis or clinical course in patients with an acute abdominal condition.
Use in Pancreatic/Biliary Tract Disease
Use morphine with caution in patients with biliary tract disease, including acute pancreatitis, as morphine sulfate may cause spasming and diminished biliary and pancreatic secretions.
Special Risk Groups
Use morphine with caution and in reduced dosages in patients with severe renal or hepatic impairment, Addison’s disease, hypothyroidism, prostatic hypertrophy, or urethral stricture, and in elderly or debilitated patients. Exercise caution in the administration of morphine sulfate to patients with CNS depression, toxic psychosis, acute alcoholism, and delirium tremens.
All opioids may aggravate convulsions in patients with convulsive disorders, and all opioids may induce or aggravate seizures.
Driving and Operating Machinery
Caution patients that morphine sulfate could impair the mental and/or physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery.
Caution patients about the potential combined effects of morphine sulfate with other CNS depressants, including other opioids, phenothiazines, sedative/hypnotics, and alcohol.
Now let’s take a closer look at the medication grid on morphine in Table 10.7a.[3],[4],[5]
Table 10.7a Morphine Medication Grid
| Class/
Subclass |
Prototype-
generic |
Administration Considerations | Therapeutic Effects | Adverse/Side Effects |
|---|---|---|---|---|
| Opioid analgesic | morphine | Given parenterally and orally
Assess pain prior to and after administration Monitor respiratory status Monitor blood pressure Assess pediatric and geriatric patients frequently Assess bowel function Use cautiously with antidepressants and other CNS depressants Naloxone is used to reverse opioid overdose |
Relieves moderate to severe pain | Respiratory depression
Confusion Hypotension Light-headedness Dizziness Sedation Constipation Nausea and vomiting Sweating |
Critical Thinking Activity 10.7a

Oral morphine was administered to a patient for rib pain (rated as “6”) from metastatic lung cancer.
When should the effectiveness of the medication be evaluated?
Note: Answers to the Critical Thinking activities can be found in the “Answer Key” sections at the end of the book.
Concerns Related to Opioid Use
CDC Guidelines for Prescribing Opioids for Chronic Pain
Improving the prescription of opioids through clinical practice guidelines can ensure patients have access to safer, effective pain treatment while also reducing the number of people who misuse or overdose from these drugs.
The CDC developed and published the CDC Guideline for Prescribing Opioids for Chronic Pain to provide recommendations for the prescribing of opioid pain medication for patients 18 and older in primary care settings. Recommendations focus on the use of opioids in treating chronic pain (pain lasting longer than 3 months or past the time of normal tissue healing) outside of active cancer treatment, palliative care, and end-of-life care.[6]
The Need
Improving the way opioids are prescribed through clinical practice guidelines can ensure patients have access to safer, more effective chronic pain treatment while reducing the risk of opioid use disorder, overdose, and death.
- More than 11.5 million Americans aged 12 or older reported misusing prescription opioids in 2016.
- An estimated 11% of adults experience daily pain.
- Millions of Americans are treated with prescription opioids for chronic pain.
- Primary care providers are concerned about patient addiction and report insufficient training in prescribing opioids.
Guideline Overview
The CDC Guideline addresses patient-centered clinical practices including conducting thorough assessments, considering all possible treatments, closely monitoring risks, and safely discontinuing opioids. The three main focus areas in the guideline include:
1. Determining when to initiate or continue opioids for chronic pain
- Selection of non-pharmacologic therapy, nonopioid pharmacologic therapy, opioid therapy
- Establishment of treatment goals
- Discussion of risks and benefits of therapy with patients
2. Opioid selection, dosage, duration, follow-up, and discontinuation
- Selection of immediate-release or extended-release and long-acting opioids
- Dosage considerations
- Duration of treatment
3. Considerations for follow-up and discontinuation of opioid therapy
- Assessing risk and addressing harms of opioid use
- Evaluation of risk factors for opioid-related harms and ways to mitigate patient risk
- Review of prescription drug monitoring program (PDMP) data
- Use of urine drug testing
- Considerations for co-prescribing benzodiazepines
- Arrangement of treatment for opioid use disorder[7]
Understanding the Epidemic
Drug overdose deaths continue to increase in the United States. From 1999 to 2017, more than 700,000 people have died from a drug overdose. Around 68% of the more than 70,200 drug overdose deaths in 2017 involved an opioid. In 2017, the number of overdose deaths involving opioids (including prescription opioids and illegal opioids like heroin and illicitly manufactured fentanyl) was 6 times higher than in 1999. On average, 130 Americans die every day from an opioid overdose.[8]
From 1999-2017, almost 400,000 people died from an overdose involving any opioid, including prescription and illicit opioids. This rise in opioid overdose deaths can be outlined in three distinct waves. See Figure 10.9 for a graphic representation of the waves of opioid deaths.[9]
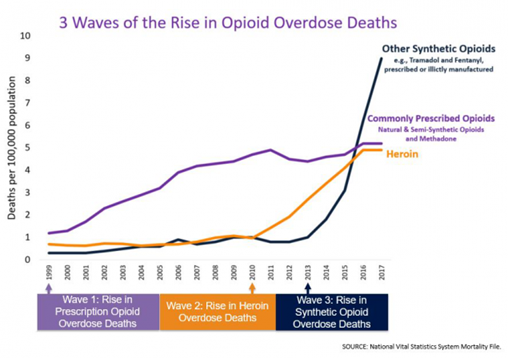
The first wave began with increased prescribing of opioids in the 1990s, with overdose deaths involving prescription opioids (natural and semi-synthetic opioids and methadone). The second wave began in 2010 with rapid increases in overdose deaths involving heroin. The third wave began in 2013, with significant increases in overdose deaths involving synthetic opioids – particularly those involving illicitly-manufactured fentanyl.[10]
Combating the Opioid Overdose Epidemic
The CDC is committed to fighting the opioid overdose epidemic and supporting states and communities as they continue work to identify outbreaks, collect data, respond to overdoses, and provide care to those in their communities. CDC’s Prevention for States and Data-Driven Prevention Initiative programs aims center around the enhancement of PDMPs within clinical and public health settings, insurer and community interventions, evaluation of state-level policies, and other innovative strategies that states can employ.
CDC’s Enhanced State Opioid Overdose Surveillance program aims to support and build the capacity of states to monitor the epidemic by improving the timeliness and quality of surveillance data focusing on both fatal and nonfatal opioid overdose.[11]
IV Drug Diversion and Impaired Health Care Workers
In every organization, drug diversion is a potential threat to patient safety. Risks to patients include inadequate pain relief and exposure to infectious diseases from contaminated needles and drugs, compounded by potentially unsafe care due to the health care worker’s impaired performance. Furthermore, diversion may cause undue suffering to patients who don’t receive analgesic relief, can be costly to an organization by damaging its reputation, and may lead to major civil and criminal monetary penalties[12] More information about surveillance programs, drug diversion, and impaired health workers is included in the “Legal/Ethical” chapter.
Download the PDF to read the full article by the Joint Commission.
Naloxone (Narcan)
Mechanism of Action
Naloxone reverses analgesia and the CNS and respiratory depression caused by opioid agonists. It competes with opioid receptor sites in the brain and, thereby, prevents binding with receptors or displaces opioids already occupying receptor sites.
Indications for Use
Naloxone is indicated for the complete or partial reversal of opioid depression, including respiratory depression induced by natural and synthetic opioids.
Nursing Considerations Across the Lifespan
Naloxone is safe for all ages.
Adverse/Side Effects
Adverse effects include tremors, drowsiness, sweating, decreased respirations, hypertension, nausea, and vomiting.
Patient Teaching & Education
Patients should be advised regarding the risks associated with opioid analgesic use and the need for opioid antagonists. Please see the outlined “Special Considerations” for usage below.[13]
Special Considerations
Postoperative
The following adverse events have been associated with the use of naloxone hydrochloride injection in postoperative patients: hypotension, hypertension, ventricular tachycardia and fibrillation, dyspnea, pulmonary edema, and cardiac arrest. Death, coma, and encephalopathy have been reported as results of these events. Excessive doses of naloxone in postoperative patients may result in significant reversal of analgesia and may cause agitation.
Opioid Depression
Abrupt reversal of opioid depression may result in nausea, vomiting, sweating, tachycardia, increased blood pressure, tremulousness, seizures, ventricular tachycardia and fibrillation, pulmonary edema, and cardiac arrest, which may result in death.
Opioid Dependence
Abrupt reversal of opioid effects in persons who are physically dependent on opioids may precipitate an acute withdrawal syndrome, which may include, but not limited to, the following signs and symptoms: body aches, fever, sweating, runny nose, sneezing, piloerection, yawning, weakness, shivering or trembling, nervousness, restlessness or irritability, diarrhea, nausea or vomiting, abdominal cramps, increased blood pressure, and tachycardia. In the neonate, opioid withdrawal may also include convulsions, excessive crying, and hyperactive reflexes.
Now let’s take a closer look at the medication grid on naloxone in Table 10.7b.[14],[15]
Table 10.7b Naloxone Medication Grid
| Class/
Subclass |
Prototype-
generic |
Administration Considerations | Therapeutic Effects | Adverse/Side Effects |
|---|---|---|---|---|
| Opioid antagonist | naloxone | Given parenterally and inhaled
Assess for reversal of opioid effect Assess for hypertension Assess for return of pain Naloxone has a shorter duration of action than opioids, and repeated doses are usually necessary |
Blocks the effects of opioid CNS and respiratory depression | Agitation
Tremors Drowsiness Sweating Decreased respirations Hypertension Nausea and vomiting |
Critical Thinking Activity 10.7b

A post-operative patient just received naloxone for respiratory depression.
When should the patient’s respiratory status be reassessed?
Note: Answers to the Critical Thinking activities can be found in the “Answer Key” sections at the end of the book.
- McCuistion, L., Vuljoin-DiMaggio, K., Winton, M, & Yeager, J. (2018). Pharmacology: A patient-centered nursing process approach. pp. 268-270, 324, 332. Elsevier. ↵
- uCentral from Unbound Medicine. https://www.unboundmedicine.com/ucentral ↵
- Frandsen, G., Pennington, S. (2018). Abrams’ clinical drug: Rationales for nursing practice (11th ed.). (pg. 305, 310, 952-953, 959-960). Wolters Kluwer. ↵
- Vallerand, A. & Sanoski, C. A. (2019). Davis’s Drug Guide for Nurses, (16th ed.). F.A. Davis Company. ↵
- This work is a derivative of Daily Med by U.S. National Library of Medicine in the public domain. ↵
- Centers for Disease Control and Prevention. (2019, August 28). Opioid overdose, CDC guideline for prescribing opioids for chronic pain. https://www.cdc.gov/drugoverdose/prescribing/guideline.html ↵
- Centers for Disease Control and Prevention. (2019, August 28). Opioid overdose, CDC guideline for prescribing opioids for chronic pain.https://www.cdc.gov/drugoverdose/prescribing/guideline.html ↵
- Centers for Disease Control and Prevention. (2018, December 19). Opioid Overdose, Understanding the Epidemic. https://www.cdc.gov/drugoverdose/epidemic/index.html. ↵
- "3 Waves of the Rise of Opioid Overdose Deaths" by National Vital Statics System, CDC is licensed under CC0 ↵
- Centers for Disease Control and Prevention. (2018, December 19). Opioid Overdose, Understanding the Epidemic. https://www.cdc.gov/drugoverdose/epidemic/index.html. ↵
- Centers for Disease Control and Prevention. (2018, December 19). Opioid Overdose, Understanding the Epidemic. https://www.cdc.gov/drugoverdose/epidemic/index.html. ↵
- The Joint Commission, Division of Healthcare Improvement. (2019). Drug diversion and impaired health care workers. Quick Safety (48). ↵
- uCentral from Unbound Medicine. https://www.unboundmedicine.com/ucentral ↵
- Frandsen, G., Pennington, S. (2018). Abrams’ clinical drug: Rationales for nursing practice (11th ed.). (pg. 305, 310, 952-953, 959-960). Wolters Kluwer. ↵
- This work is a derivative of Daily Med by U.S. National Library of Medicine in the public domain. ↵
To receive the opioid, the patient pushes a button on the PCA device, which releases a specific dose but also has a lockout mechanism to prevent an overdose. Included with with hydromorphone or fentanyl.

