1.5 Metabolism
Metabolism
After a drug has been absorbed and distributed throughout the body, it is broken down by a process known as metabolism so that it can be excreted from the body. Drugs undergo chemical alteration by various body systems to create compounds that are more easily excreted.
As previously discussed in this chapter, medications that are swallowed or otherwise administered into the gastrointestinal tract are inactivated by the intestines and liver, known as the first-pass effect. Additionally, everything that enters the bloodstream, whether swallowed, injected, inhaled, absorbed through the skin, or produced by the body itself, is metabolized by the liver. See Figure 1.5[1] for an image of the liver. These chemical alterations are known as biotransformations. The biotransformations that take place in the liver are performed by liver enzymes.
Biotransformations occur by mechanisms categorized as either Phase I (modification), Phase II (conjugation), and in some instances, Phase III (additional modification and excretion.)[2]
Phase I biotransformations alter the chemical structure of the drug. Many of the products of enzymatic breakdown, called metabolites, are less chemically active than the original molecule. For this reason, the liver is referred to as a “detoxifying” organ. An example of a Phase I biotransformation is when diazepam, a medication prescribed for anxiety, is transformed into desmethyldiazepam and then to oxazepam. Both these metabolites produce similar physiological and psychological effects of diazepam.[3]
In some instances, Phase I biotransformations change an inactive drug into an active form called a “prodrug.” Prodrugs improve a medication’s effectiveness. They may also be designed to avoid certain side effects or toxicities. For example, sulfasalazine is a medication prescribed for rheumatoid arthritis. It is prodrug that is not active in its ingested form but becomes active after Phase I modification.
Phase II biotransformations involve reactions that couple the drug molecule with another molecule in a process called conjugation. Conjugation typically renders the compound pharmacologically inert and water-soluble so it can be easily excreted. These processes can occur in the liver, kidney, lungs, intestines, and other organ systems. An example of Phase II metabolism is when oxazepam, the active metabolite of diazepam, is conjugated with a molecule called glucuronide so that it becomes physiologically inactive and is excreted without further chemical modification.[4]
Following Phase II metabolism, Phase III biotransformations may also occur, where the conjugates and metabolites are excreted from cells.[5]
Factors Affecting Metabolism
Critical factors in drug metabolism are the type and concentration of liver enzymes. The most important enzymes for medical purposes are monoamine oxidase and cytochrome P450. These two enzymes are responsible for metabolizing dozens of chemicals.[6]
Drug metabolism can be influenced by a number of factors. One major disruptor of drug metabolism is depot binding. Depot binding is the coupling of drug molecules with inactive sites in the body, resulting in the drug not being accessible for metabolism. This action can also affect the duration of action of other medications susceptible to depot binding. For example, tetrahydrocannabinol (THC), the main psychoactive component of marijuana, is highly lipid-soluble and depot binds in the adipose tissue of users. This interaction drastically slows the metabolism of the drug, so metabolites of THC can be detected in urine weeks after the last use.[7]
Another factor in drug metabolism is enzyme induction. Enzymes are induced by repeated use of the same drug. The body becomes accustomed to the constant presence of the drug and compensates by increasing the production of the enzyme necessary for the drug’s metabolism. This contributes to a condition referred to as tolerance and causes clients to require ever-increasing doses of certain drugs to produce the same effect. For example, clients who take opioid analgesics over a long period of time will notice that their medication becomes less effective over time.[8]
In contrast, some drugs have an inhibitory effect on enzymes, making the client more sensitive to other medications metabolized through the action of those enzymes. For example, monoamine oxidase inhibitors (MAOIs) are prescribed as antidepressants because they block monoamine oxidase, the enzyme that breaks down serotonin and dopamine, thus increasing the concentration of these chemicals in the central nervous system. However, this can cause problems when clients taking an MAOI also take other medications that increase the levels of these chemicals, such as dextromethorphan found in cough syrup.[9]
Additionally, drugs that share metabolic pathways can “compete” for the same binding sites on enzymes, thus decreasing the efficiency of their metabolism. For example, alcohol and some sedatives are metabolized by the cytochrome P450 enzyme and only a limited number of these enzymes exist to break these drugs down. Therefore, if a client takes a sedative after drinking alcohol, the sedative is not well-metabolized because most of cytochrome P450 enzymes are filled by alcohol molecules. This results in reduced excretion and high levels of both drugs in the body with enhanced effects. For this reason, the co-administration of alcohol and sedatives can be deadly.
Clinical Significance
When administering medication, nurses must know how and when the medication is metabolized and eliminated from the body. Most of the time, the rate of elimination of a drug depends on the concentration of the drug in the bloodstream. However, the elimination of some drugs occurs at a constant rate that is independent of plasma concentrations. For example, the ethanol contained in alcoholic beverages is eliminated at a constant rate of about 15 mL/hour regardless of the concentration in the bloodstream.[10]
Half Life
Half-life refers to the rate at which 50% of a drug is eliminated from the body. Half-life can vary significantly between drugs. Some drugs have a short half-life of only a few hours and must be given multiple times a day, whereas other drugs have half-lives exceeding 12 hours and can be given as a single dose every 24 hours. See Figure 1.6[11] for an illustration of half-life affecting the blood concentration of medication over time.
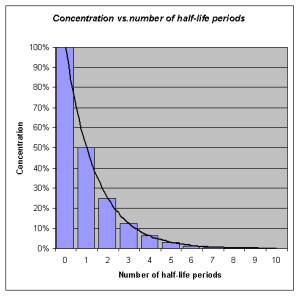
Half-life affects the duration of the therapeutic effect of a medication. Many factors can influence half-life. For example, liver disease can prolong half-life if it is no longer effectively metabolizing the medication. Information about half-life of a specific medication can be found in evidence-based medication references. For example, in the “Clinical Pharmacology” section of the DailyMed reference for furosemide, the half-life is approximately two hours.
Depending on whether a drug is metabolized and eliminated by the kidneys or liver, impairment in either of these systems can significantly alter medication dosing, frequency of doses, anticipated therapeutic effect, and even whether a particular medication can be used at all. Nurses must work with other members of the health care team to prevent drug interactions that could significantly affect a client’s health and well-being. Nurses must be alert for signs of a toxic buildup of metabolites or active drugs, particularly if the client has liver or kidney disease, so that they can alert the health care provider. In other cases, drugs such as warfarin and certain antibiotics are dosed and monitored by pharmacists, who monitor serum levels of the drugs, as well as kidney function.
Life Span Considerations
Neonate & Pediatric
The developing liver in infants and young children produces decreased levels of enzymes. This may result in a decreased ability of the young child or neonate to metabolize medications. In contrast, older children may experience increased metabolism and require higher doses of medications once the hepatic enzymes are fully produced.[12]
Older Adult
Metabolism by the liver may significantly decline in the older adult. As a result, dosages should be adjusted according to the client’s liver function and their anticipated metabolic rate. First-pass metabolism also decreases with aging, so older adults may have higher “free” circulating drug concentrations and thus be at higher risk for side effects and toxicities.[13]
Critical Thinking Activity 1.5
Metabolism can be influenced by many factors within the body. If a client has liver damage, they may not be able to breakdown (metabolize) medications as efficiently. Dosages are calculated according to the liver’s ability to metabolize and the kidney’s ability to excrete.
When caring for a client with cirrhosis, how can this condition impact the dosages prescribed?
Note: Answers to the Critical Thinking activities can be found in the “Answer Key” section at the end of the book.
Did You Know?
Did you know that, in some people, a single glass of grapefruit juice can alter levels of drugs used to treat allergies, heart diseases, and infections? Fifteen years ago, pharmacologists discovered this “grapefruit juice effect” by luck, after giving volunteers grapefruit juice to mask the taste of a medicine. Nearly a decade later, researchers figured out that grapefruit juice affects the metabolizing rates of some medicines by lowering levels of a drug-metabolizing enzyme called CYP3A4 (part of the CYP450 family of drug-binding enzymes) in the intestines.
Paul B. Watkins of the University of North Carolina at Chapel Hill discovered that other juices like Seville (sour) orange juice—but not regular orange juice—have the same effect on the liver’s ability to metabolize using enzymes. Each of ten people who volunteered for Watkins’ juice-medicine study took a standard dose of felodopine, a drug used to treat high blood pressure, diluted in grapefruit juice, sour orange juice, or plain orange juice. The researchers measured blood levels of felodopine at various times afterward. The team observed that both grapefruit juice and sour orange juice increased blood levels of felodopine, as if the people had received a higher dose. Regular orange juice had no effect. Watkins and his coworkers have found that a chemical common to grapefruit and sour oranges, dihydroxybergamottin, is likely the molecular culprit. Thus, when taking medications that use the CYP3A4 enzyme to metabolize, clients are advised to avoid grapefruit juice and sour orange juice.[14]

- “Liver Hepatic Organ Jaundice Bile Fatty Liver - Liver” by VSRao is licensed under CC0 ↵
- This work is a derivative of StatPearls by Susa, Hussain, and Preuss and is licensed under CC BY 4.0 ↵
- This work is a derivative of StatPearls by Susa, Hussain, and Preuss and is licensed under CC BY 4.0 ↵
- This work is a derivative of StatPearls by Susa, Hussain, and Preuss and is licensed under CC BY 4.0 ↵
- This work is a derivative of StatPearls by Susa, Hussain, and Preuss and is licensed under CC BY 4.0 ↵
- This work is a derivative of StatPearls by Susa, Hussain, and Preuss and is licensed under CC BY 4.0 ↵
- This work is a derivative of StatPearls by Susa, Hussain, and Preuss and is licensed under CC BY 4.0 ↵
- This work is a derivative of StatPearls by Susa, Hussain, and Preuss and is licensed under CC BY 4.0 ↵
- This work is a derivative of StatPearls by Susa, Hussain, and Preuss and is licensed under CC BY 4.0 ↵
- This work is a derivative of StatPearls by Susa, Hussain, and Preuss and is licensed under CC BY 4.0 ↵
- “Concentration_vs_number_of_half-life_periodes.png” by OPPSD is licensed under CC BY-SA 3.0 ↵
- Fernandez, E., Perez, R., Hernandez, A., Tejada, P., Arteta, M., & Ramos, J. T. (2011). Factors and mechanisms for pharmacokinetic differences between pediatric population and adults. Pharmaceutics, 3(1), 53–72. https://doi.org/10.3390/pharmaceutics3010053 ↵
- Fernandez, E., Perez, R., Hernandez, A., Tejada, P., Arteta, M., & Ramos, J. T. (2011). Factors and mechanisms for pharmacokinetic differences between pediatric population and adults. Pharmaceutics, 3(1), 53–72. https://doi.org/10.3390/pharmaceutics3010053 ↵
- This work is a derivative of Medicines by Design by US Department of Health and Human Services, National Institutes of Health, National Institute of General Medical Sciences and is available in the Public Domain. ↵
The breakdown of a drug molecule.
The rate at which 50% of a drug is eliminated from the bloodstream.

