1.1 Gas Laws
Key Gas Laws and Concepts for Respiratory Therapists
Understanding gas laws is essential for respiratory therapists, as these principles directly impact patient care. Respiratory therapists constantly work with gases, whether to deliver medications, drive respiratory equipment, or titrate gases to meet the physiological needs of the patient. Below is an overview of relevant gas laws, equations, and other key terms to support your practice.
Gas Laws and Equations
Boyle’s Law
Boyle’s law states, at constant temperature and mass, the volume of a gas varies inversely with pressure. In a given system, as the pressure goes up, the volume will go down. This is a concept that has application in the measurement of lung capacities and volumes in a body plethysmography box in a pulmonary function laboratory (see Figure 1[1]).

Therefore, if we have a container at a known pressure and volume, then the pressure changes. Utilizing basic algebra you can solve for the unknown volume. The algebra formula is as follows:
[latex]V2 = \frac {P1V1}{P2}[/latex]
Example
You are in a PFT (pulmonary function test) laboratory with a patient. The initial pressure in the body plethysmography box is 1500 psi, and the volume is 420 mL. If the patient returns a several days later and the new pressure in the body plethysmography box is 1000 psi, solve for the new volume.
[latex]V2 = \frac {P1V1}{P2}[/latex]
[latex]V2 = \frac {1500 psi \times 420 mL}{1000 mL}[/latex]
Charles’s Law
Charles’s law states, at constant pressure and mass, the volume of a gas varies directly with temperature. You should remember that Kelvin is the only linear temperature scale, and all temperatures must be converted to Kelvin.
[latex]V1=T1 = V2T2[/latex]
Utilizing basic algebra you can solve for the second volume. The algebra formula is as follows:
[latex]V2 = \frac {V1T2}{T1}[/latex]
View the following supplementary YouTube video[2] that explains Charles’ law in detail: CHARLES’ Law | Animation
Gay-Lussac’s Law
Gay-Lussac’s law states, at constant volume and mass, the pressure of a gas varies directly with temperature.
[latex]P1T1 = P2T2[/latex]
Utilizing basic algebra you can solve for the second pressure (P2). The algebra formula is as follows:
[latex]P2 = \frac {P1T2}{T1}[/latex]
View the following supplementary YouTube video[3] that explains Gay-Lussac’s Law in detail: Chemistry: Gay-Lussac’s Law (Gas Laws) with 2 Example Problems
Dalton’s Law of Partial Pressures
The total pressure of a gas mixture is the sum of the partial pressures of each constituent gas. This gas law is important in several contexts in the clinical experience of the respiratory therapist. A patient who is placed in a hyperbaric chamber is breathing supra-atmospheric (in other words, giving higher than atmospheric pressure) pressurized O2 to enhance wound healing. In determining the oxygen content inside the lung structure of the alveolus, the different primary gas pressures are accounted for so when the math is complete, the respiratory therapist can accurately determine the PAO2 in the alveolar tissue of the lung.
- The partial pressure of each gas is what it would exert if alone.
- Each partial pressure is proportional to the percentage of the gas in the mixture.
Partial Pressure of a Gas
The partial pressure of a gas can be calculated using Dalton’s law, which states the gas pressure in a system is the sum of the independent gasses (Ptotal = PH2O + PAO2 + PN2). In other words, the total is the sum of the parts.
View the following supplementary YouTube video[4] that explains Dalton’s Law in detail: Breathing 5- Gas Laws
Key Terms for Respiratory Therapy to Use With Gas Laws
- BTPS: Body temperature and pressure, saturated (conditions within the lungs)
- ATPS: Ambient temperature and pressure, saturated
- STPD: Standard temperature (0°C) and pressure (760 mm Hg), dry
Note: Water vapor exerts 47 mm Hg at 37°C. This is important for airway humidification to be considered at a later juncture.
Composition of Atmospheric Gases
It is important for respiratory therapists to know the primary gases in Earth’s atmosphere. These gasses include the following:
- Nitrogen (N2): 78.08%
- Oxygen (O2): 20.95%
- Argon (Ar): 0.93%
- Carbon Dioxide (CO2): 0.03%
- Trace elements: 0.01%
Diffusion and Solubility Laws
Graham’s Law
Graham’s law states that the rate of diffusion of a gas into a liquid is inversely proportional to the square root of its density; denser gases diffuse more slowly. Conversely, a lighter gas, such as helium, will diffuse more quickly.
Henry’s Law
Henry’s law states that the amount of gas dissolving in a liquid at a given temperature is proportional to the partial pressure of that gas.
- Gases with higher partial pressures dissolve more readily in liquids.
- Although oxygen is less dense, carbon dioxide has a much higher solubility coefficient, resulting in CO2 diffusing 19 times faster than O2.
- Graham and Henry’s law combine to demonstrate that there is 20 times more solubility of CO2 compared to O2 in the blood.
Fick’s Law
In its simplest form, Fick’s law identifies the diffusion across a permeable membrane. The thicker the membrane, the less diffusion occurs. Graham’s law and Henry’s law together form the basis for the diffusion coefficient used in the Fick’s law equation.
Flow and Related Principles
Flow is the movement of a gas volume from one place to another over time and typically measured in liters per minute (L/min).
Flow can be increased by the following:
- Increasing the pressure gradient
- Increasing the size of the opening or reducing resistance or opposition to flow
Bernoulli Principle
The Bernoulli principle states that as the forward velocity of a gas increases, its lateral pressure decreases, with a corresponding increase in forward pressure. This is important because it explains the behavior of airflow through the airways such as the bronchi and the bronchioles. As air moves through the constricted parts of the respiratory system, like the bronchi and bronchioles, the velocity increases, causing a decrease in pressure[5].
Venturi Principle
The Venturi Principle suggests that by adding a gradually widening tube (funnel) after a jet orifice and keeping the angulation below 15°, lateral pressure can be restored to pre-jet levels (see Figure 2[6]).
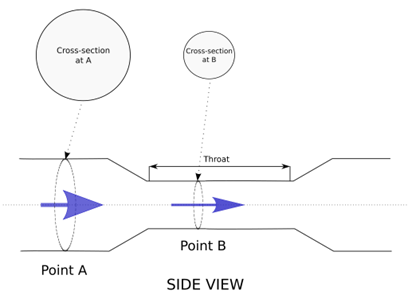
Effects of Resistance to Flow (i.e., “Back Pressure”) and Obstruction
An obstruction downstream of an air entrainment device creates back pressure, which reduces entrainment of air and total flow given to the patient, and also increases oxygen concentration. A therapist will want to ensure that there is not a downstream obstruction as the device will not work properly and the patient could be harmed.
Entrainment System
The amount of entrainment (air intake) is influenced by the following:
- Jet orifice size: Smaller orifices increase entrainment of air and total flow delivered to patient and reduce oxygen concentration.
- Entrainment port size: Larger ports increase entrainment of air and total flow delivered to patient and reduces oxygen concentration.
Poiseuille’s Law
Poiseuille’s law is critically important as it reflects the change in resistance in the lung in opposition to gas flow. This is true of the gas flow in the small airways of the lung, where the flow in the larger airways is a mixture of a linear flow and a turbulent flow, which is advantageous for the body to mobilize secretions.
The pressure needed to drive a gas (overcome airway resistance) increases if the following occurs:
- The length of the tube increases.
- The viscosity of the gas increases.
- The flow rate of the gas increases.
- The radius of the tube decreases.
View the following supplementary YouTube video[7] that explains Poiseuille’s law in detail: Poiseuille’s law
Reynold’s Number and Turbulent Flow
The Reynolds number is the ratio of inertial forces to viscous forces within a fluid/gas that is subjected to relative internal movement due to different fluid/gas velocities. Gas flow is more likely to become turbulent when the following occur:
- Gas density is high.
- Flow rate is high.
- Tube diameter is large.
- Gas viscosity is low.
Turbulent flow typically begins when the Reynold’s number exceeds 2,000.
- “Body_plethysmograph_box” by Stan3000 is licensed under CC BY-SA 3.0 ↵
- EarthPen. (2020, October 12). CHARLES' law | animation [Video]. YouTube. All rights reserved. https://www.youtube.com/watch?v=twCcSsHmMgl ↵
- Socratica. (2015, February 4). Chemistry: Gay-Lussac's law (gas laws) with 2 example problems [Video]. YouTube. All rights reserved. https://www.youtube.com/watch?v=0Oq7bCSDPxE ↵
- Wendy Riggs. (2015, March 17). Breathing 5- gas laws [Video]. YouTube. All rights reserved. https://www.youtube.com/watch?v=q9eB-AYneA0 ↵
- Landry, J. (2024). The 19+ laws of the lungs and respiratory system (2025). Respiratory Therapy Zone. https://www.respiratorytherapyzone.com/respiratory-system-laws/ ↵
- “Venturi-nomaths” by unknown author is licensed under CC BY 3.0. ↵
- Mechanisms and Logic in Human Physiology. (2022, September 7). Poiseuille's law [Video]. YouTube. All rights reserved. https://www.youtube.com/watch?v=r1_Uo9pGwy8 ↵

