17.5 Applying the Nursing Process and Clinical Judgment Model to Congenital Heart Defects
This section will apply the nursing process and the NCSBN Clinical Judgment Measurement Model (NCJMM) to caring for infants and children with congenital heart defects. The steps of the NCJMM are indicated in parentheses next to the stages of the nursing process.
Assessment (Recognize Cues)
Signs of serious congenital heart defects (CHD) typically manifest early in infancy. However, signs of less serious defects may begin anytime during the newborn period, infancy, or early childhood. Depending upon the severity of the condition, signs may not occur until complications of heart failure or pulmonary hypertension develop. Nurses may detect subtle cues of a congenital heart defect in infants and children during a routine assessment.
Assessment begins with a thorough health history, obtained by interviewing the parent(s) or main caregiver, followed by a physical examination. Subtle cues of CHD in an infant who has not been previously diagnosed with a CHD include parents reporting their infant is having difficulty feeding, takes longer to feed, or appears hungry or irritable soon after feeding. Additionally, the infant/child may demonstrate mild tachypnea at rest and demonstrate failure to gain weight over time. These cues require a focused assessment for CHD. See Table 17.5a for focused objective and subjective assessment data related to congenital heart defects.[1]
Table 17.5a. Focused Objective and Subjective Assessment Data Related to Congenital Heart Defects[2],[3]
*Note that bolded information should be promptly reported to the health care provider.
| Assessment Type | Sample Interview Questions and Assessments |
|---|---|
| Health history |
|
| Inspection |
*A significant difference in upper extremity versus lower extremity color indicates a potential restriction in blood flow and should be promptly reported to the provider.
*Tachypnea, sternal or intercostal retractions, or nasal flaring indicate respiratory distress and should be promptly reported to the provider.
*Lethargy, limpness, loss of consciousness, or asymmetrical movements indicate potential complications such as stroke or shock. Emergency assistance should be obtained while the provider is promptly notified. |
| Auscultation |
*S3 indicates potential fluid overload and should be promptly reported to the provider. S4 can indicate reduced ventricular compliance. Murmurs can indicate incomplete closure of a PFO, ASD, or VSD. A rub sound can indicate pericarditis (irritation of the pericardium). |
| Palpation |
*A significant difference in quality of upper extremity versus lower extremity pulse or temperature indicates a potential restriction in blood flow and should be promptly reported to the provider. |
View a supplementary YouTube video[5] on cardiac examination of the newborn by Stanford Health: Cardiac Examination of the Newborn.
Physical assessment findings for diagnosed congenital heart defects vary depending on type and severity of the defect and the presence of complications. Assessment of a child diagnosed with a cyanotic CHD includes assessment of baseline perfusion/cyanosis, respiratory status, and general appearance. See Table 17.5b for potential clinical manifestations of congenital heart defects categorized by acyanotic or cyanotic conditions (as previously discussed in the “Categories of Congenital Heart Defects – Acyanotic and Cyanotic Defects” section).
Table 17.5b. Potential Clinical Manifestations of Congenital Heart Defects[6],[7],[8],[9],[10],[11],[12]
*Note: Bolded assessment findings indicate critical findings requiring immediate intervention and/or provider notification.
| Body System | Acyanotic Defects (e.g., PDA, ASD, VSD) | Cyanotic Defects (e.g., ToF, TGA, HLHS, coarctation of the aorta, mitral valve stenosis) |
|---|---|---|
| Neurological |
|
|
| Cardiovascular |
|
|
| Respiratory |
|
|
| General |
|
|
Listen to normal heart sounds and extra heart sounds in the “Cardiovascular Assessment” section of the “Cardiovascular Assessment” chapter in Open RN Nursing Skills, 2e.
Diagnostic Testing
The diagnosis of congenital heart defects typically involves a combination of clinical evaluation, laboratory tests, and imaging studies. These tests help confirm the presence of CHDs, identify the severity of the defect and associated complications, and guide treatment. See Table 17.5c for a list of diagnostic tests that are helpful in identifying congenital heart defects, along with rationales for their use.
Table 17.5c. Diagnostic Tests for Diagnosing Congenital Heart Defects and Rationale[13]
| Diagnostic Test | Rationale |
|---|---|
| Electrocardiogram (ECG) | Provides a reading of electrical activity of the heart through electrodes placed across the chest. Shows heart rate, rhythm, arrhythmias, and any other normal or abnormal electrical activity. |
| Holter Monitor | A portable ECG device that the client wears that records electrical activity of the heart over a period of time, usually 24-48 hours, on an outpatient basis. The client or parent presses a button to record events such as shortness of breath or palpitations. The recording shows arrhythmias and any other normal or abnormal electrical activity that occurs during these recorded events. |
| Chest X-ray (radiograph) | A radiographic image of the chest that shows normal anatomy, as well as any abnormal findings such as pulmonary congestion, abnormal heart location, size, or shape. |
| Echocardiogram (Echo) | An ultrasound of the heart, which may be obtained through the chest wall (transthoracic), or via a probe inserted in the esophagus (transesophageal) that shows blood flow through the heart. Echocardiograms can demonstrate oxygen-rich and oxygen-poor blood flow, regurgitation, valvular dysfunction, ejection fraction, septal defects, or myocardial hypertrophy. An echocardiogram may also be performed during heart surgery directly on the heart muscle or while the fetus is still in utero. |
| Magnetic Resonance Imaging (MRI) | Imaging obtained by placing the infant or child in a machine that emits a strong magnetic field that surrounds the body. This imaging takes an hour or more and allows visualization of any cardiac shunts, valve function, heart structures, and thickness of myocardial walls. |
| Ventilation-Perfusion Scan (VQ scan) | Involves inhalation of a radioactive gas to examine the airways, followed by injection of an isotope intravenously, which is then scanned and tracked to determine blood flow and flow patterns through the lung vessels. |
| Pulse Oximetry Screening Test | A pulse oximetry screening test is performed on newborns at 24 hours of age to assess for critical congenital heart defects (CCHD). The pulse oximeter probe is applied to the infant or child’s finger on the right hand (preductal) and either foot (postductal). If the readings are less than 95% or there is more than a 3% difference between the two readings, additional diagnostic testing for CHD is required. This test screens for ToF, coarctation of the aorta, hypoplastic left heart syndrome, pulmonary atresia, d-Transposition of the great arteries, and persistent pulmonary hypertension.[14] |
| Cardiac Catheterization | An invasive procedure that involves insertion of a catheter into a vein or artery that is threaded into the heart, allowing measurement of heart and lung pressures and evaluation of blood flow through the heart through the use of dye. Cardiac output can be measured, as well as mapping of the electrical conduction system to evaluate where arrhythmias may be originating. Cardiac catheterization also allows for correction of some heart defects. |
Diagnoses (Analyze Cues)
Nursing priorities for clients with congenital heart defects or congenital heart disease include symptom management, monitoring for complications, and minimizing distress. Nursing diagnoses for clients with CHDs should be created based on specific client needs, signs and symptoms they are exhibiting, and the etiology of the problem, meaning the specific defect. Nursing diagnoses guide the creation of client specific care plans, including client outcomes, nursing interventions, and evaluation of the outcomes to enable provision of comprehensive care.
Possible nursing diagnoses for clients with congenital heart defects or congenital heart disease include the following[15]:
- Deficient knowledge r/t specific congenital heart defect present
- Imbalanced nutrition: less than body requirements r/t decreased oral intake
- Decreased cardiac output r/t altered afterload
- Excess fluid volume r/t impaired cardiopulmonary function
- Risk for ineffective breathing pattern r/t increased pulmonary resistance
Outcome Identification (Generate Solutions)
Outcome identification involves setting short- and long-term goals and creating expected outcome statements tailored to the client’s specific needs. These outcomes should be measurable and responsive to nursing interventions. Sample outcomes for selected nursing diagnoses are as follows[16]:
- The client’s family or caregivers will state two appropriate actions to take during a hypercyanotic episode by the end of teaching.
- The client will maintain weight fluctuations of less than 0.5 kg per day throughout hospitalization.
- The client will maintain cardiac output within normal limits as evidenced by heart rate and systolic blood pressure in normal range for age throughout hospitalization.
- The client’s lungs will remain clear throughout hospitalization.
- The client will maintain a breathing pattern that supports adequate oxygenation throughout hospitalization.
Implementation (Take Action)
Medical Interventions
Several medications may be prescribed to treat or manage symptoms associated with congenital heart defects and/or disease. Common medication classes are discussed in the following subsections.
Non-Steroidal Anti-inflammatory Drugs (NSAIDS)
Non-steroidal anti-inflammatory drugs (NSAIDS) such as ibuprofen or indomethacin may be prescribed for a patent ductus arteriosus. When administered soon after birth in term and premature infants, it triggers the PDA to constrict and close the opening. Dosage may be repeated in the event that the shunt is not completely closed with initial drug administration, although effectiveness decreases with repeated doses. NSAIDS, specifically ibuprofen, have a PDA closure efficacy rate of approximately 70-85%. It is important to note that NSAIDS carry risks of adverse events in neonatal and infant populations, including renal failure, thrombocytopenia, necrotizing enterocolitis, intestinal bleeding or perforation, cerebral hypoperfusion, and hyperbilirubinemia. Most of these adverse effects are related to vasoconstrictive effects of NSAIDS.[17],[18],[19]
NSAIDS and prostaglandins inhibitors are contraindicated in neonates with defects such as Tetralogy of Fallot and severe coarctation of the aorta that rely on a patent ductus arteriosus to pass oxygenated blood into either systemic circulation or to maintain pulmonary circulation. In these instances, closure of the ductus arteriosus may result in severe hypoxemia, cyanosis, systemic hypoperfusion, shock, and death if the defect is not surgically corrected prior to PDA closure. With critical defects that depend on patency of the ductus arteriosus, prostaglandin (PEG1) is administered as a temporary measure to maintain the PDA until surgical intervention can be performed. PEG1 helps relax the ductus arteriosus and keep it dilated enough for blood to flow through. PEG1 is rapidly metabolized by the lungs and, as such, must be administered by continuous intravenous infusion.[20]
Review information about NSAIDS in the “Nonopioid Analgesics” section of the “Analgesics & Musculoskeletal System” chapter in Open RN Nursing Pharmacology, 2e.
Prostaglandins Inhibitors
Acetaminophen, a prostaglandins inhibitor, may be prescribed to close a patent ductus arteriosus (PDA). Prostaglandins cause dilation of the opening in a PDA, so inhibiting prostaglandins cause the opening to constrict and ideally close. Studies have reported PDA closure rates ranging from 10-80% after acetaminophen administration. Acetaminophen has much lower risk of side effects and adverse events than do NSAIDS.[21],[22],[23]
Review information about acetaminophen in the “Nonopioid Analgesics” section of the “Analgesics & Musculoskeletal System” chapter in Open RN Nursing Pharmacology, 2e.
Cardiac Glycosides
Digoxin is a cardiac glycoside used to improve cardiac output while also controlling heart rate and rhythm. Digoxin improves cardiac output by increasing contractility (the force of the cardiac contraction), known as having a positive inotropic effect. Digoxin slows heart rate by inhibiting atrioventricular (AV) node conduction, resulting in a slower ventricular response to electrical stimulation. This is known as having a negative chronotropic effect. Due to these cardiac effects, digoxin can be helpful in managing heart failure symptoms in infants and pediatric clients by prolonging ventricular filling times, improving cardiac output, reducing blood backup in the lungs, and increasing systemic perfusion.[24]
Digoxin also improves perfusion of the kidneys, which enhances filtration and allows the kidneys to excrete additional fluid. An infant or child may receive an initial loading dose of 20-40 mcg/kg over 24 hours to rapidly raise serum digoxin levels, called digitalization. Once digitalization is achieved, it is followed by a maintenance dose of 8-10 mcg/kg/day, given in two divided doses (4-5 mcg/kg per dose). Dosages are decreased if there is impaired renal function. Dosage is also adjusted based on serum digoxin levels that are obtained six hours after administration of a prior dose.[25]
Adverse effects such as nausea and vomiting, bradycardia, and dysrhythmias can indicate digoxin toxicity, which can be fatal. Nurses must know the acceptable dosage range for infants and children, because a small amount of excess digoxin can result in severe adverse effects, including dysrhythmias and death. Prior to administration of digoxin, the nurse auscultates the client’s apical pulse for one minute. The dose is withheld, and the provider is notified for an apical heart rate less than 100 beats per minute in infants. For older children, nurses follow parameters established by the health care provider for heart rate. Hypokalemia and hypomagnesemia cause a lower threshold for digoxin toxicity, so it is crucial for nurses to monitor potassium and magnesium levels, especially if the client is concurrently receiving diuretic medications because they can deplete electrolytes.[26],[27],[28]
Review information about digoxin in the “Cardiac Glycosides” section of the “Cardiovascular & Renal Systems” chapter in Open RN Nursing Pharmacology, 2e.
Diuretics
Diuretics such as furosemide may be prescribed to help relieve pulmonary congestion or decrease fluid overload that occurs with heart failure. Recall that heart failure can be a complication of valve stenosis or acyanotic or cyanotic defects, and, as such, diuretics may be prescribed with any of these defects. Diuretics inhibit sodium reabsorption in the kidney tubules, which, in turn, prevents water reabsorption, so excess fluid is excreted by the kidneys as urine. Increased urine output decreases overall blood volume, preload, and blood pressure. Nurses closely monitor the child’s weight and urinary output, as well laboratory results, including kidney function and electrolyte levels, specifically potassium. Clients taking diuretics may experience worsening kidney function and depletion of potassium, magnesium, or sodium.[29],[30],[31]
Review information about “Diuretics” in the “Cardiovascular & Renal Systems” chapter in Open RN Nursing Pharmacology, 2e.
Calcium Channel Blockers
Calcium channel blockers affect the calcium channels of smooth muscles, causing them to relax. This results in vasodilation of peripheral blood vessels, thereby reducing preload and afterload, with a subsequent decrease in blood pressure as systemic vascular resistance is reduced. Calcium channel blockers also have a negative inotropic effect, meaning they decrease contractility, resulting in decreased cardiac output. This effect reduces oxygen demand of cardiac muscle.[32]
Calcium channel blockers are generally not recommended for children under one year of age due to negative inotropic effects, as well as risks from affecting the developing sarcoplasmic reticulum (SR) in cardiac muscle. The SR is is responsible for releasing and storing calcium ions in muscle fibers and affects the contractility of the heart.[33] The negative inotropic effects of calcium channel blockers are contraindicated for infants experiencing heart failure, because the heart is already not pumping effectively, and this medication will further decrease the cardiac output by decreasing the heart rate.[34] The exception to this guideline is the use of nicardipine, which is used safely and effectively in infants to reduce blood pressure postoperatively after certain surgeries where strict blood pressure control is essential, such as repair of coarctation of the aorta. Recall that with coarctation of the aorta the aorta is drastically reduced in size at the area of the coarctation, resulting in increased afterload with associated blood pressure. After surgical repair, up to 30% of infants continue to have hypertension, necessitating the potential use of nicardipine postoperatively.[35]
Review information about calcium channel blockers in the “Antiarrhythmics” section of the “Cardiovascular & Renal Systems” chapter in Open RN Nursing Pharmacology, 2e.
Angiotensin Converting Enzyme (ACE) Inhibitors
Angiotensin converting enzyme (ACE) inhibitors block the conversion of Angiotensin I to Angiotensin II in the renin-angiotensin-aldosterone system, resulting in vasodilation. They also block aldosterone (a potent vasoconstrictor), resulting in sodium and water excretion by the kidneys. ACE inhibitors such as enalapril may be prescribed for children with mitral or aortic valve regurgitation (leaky valves), left to right shunts (such as atrial and ventricular septal defects), or heart failure. Vasodilation causes decreased systemic vascular resistance, resulting in decreased blood pressure. Additionally, the heart does not have to pump as hard to eject blood to the body, so hypertrophy of the cardiac muscle is prevented. By decreasing systemic vascular resistance, left-sided heart pressures are also lowered, which decreases the shunting of blood from the left to right side of the heart in clients with acyanotic cardiac defects.[36]
Review information about the renin-angiotensin-aldosterone system in the “Basic Concepts of the Cardiovascular and Renal Systems” section of the “Cardiovascular & Renal Systems” chapter in Open RN Nursing Pharmacology, 2e.
Review information about ACE inhibitors in the “Antihypertensives” section of the “Cardiovascular & Renal Systems” chapter of Open RN Nursing Pharmacology, 2e.
Vasodilators
Vasodilators relax blood vessels. Certain vasodilators, such as phosphodiesterase-5 inhibitors (sildenafil and tadalafil), endothelin receptor antagonists, and prostacyclins, specifically dilate the pulmonary arteries and decrease the pressures in the lungs. This action reduces pulmonary hypertension, promotes easier blood flow through the lungs, and may be beneficial with managing other defects contributing to pulmonary hypertension.[37],[38] However, vasodilators cause vasodilation throughout the body and may cause hypotension.[39]
A small amount of inhaled nitric oxide gas is a vasodilator that may be administered with oxygen therapy to dilate pulmonary arteries and decrease pulmonary hypertension. The benefit with using inhaled nitric oxide is that it is a selective and fast-acting vasodilator, so its effects stay within the pulmonary arteries. Nitric oxide may be used for a few days to treat pulmonary hypertension, but is not a long-term treatment. Studies have found that while nitric oxide is beneficial in dilating pulmonary arteries, it does not necessarily reduce length of hospital stay or mortality risks associated with persistent pulmonary hypertension of the newborn.[40],[41]
Review information about phosphodiesterase-5 inhibitors in the “Erectile Agents” section of the “Cardiovascular & Renal Systems” chapter in Open RN Nursing Pharmacology, 2e.
Blood Coagulation Modifiers
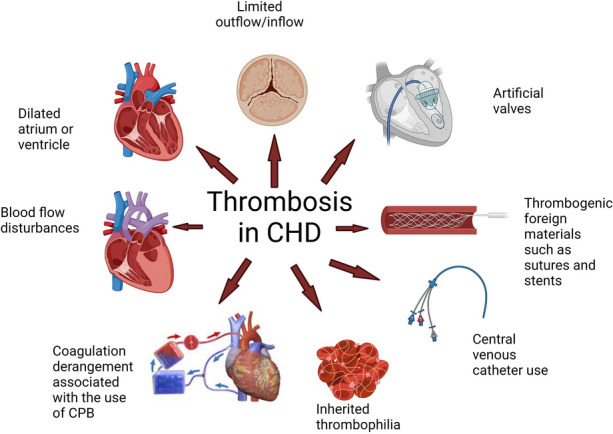
Clients with congenital heart defects are at a high risk of thrombosis (blood clots) that contribute to increased morbidity and mortality. With many heart defects, blood flow is turbulent or there is restricted blood flow, causing blood stasis. Stasis allows blood to pool in certain areas with formation of clots. Additional risk factors for thrombosis include heart failure, arrhythmias, increased viscosity of the blood with polycythemia, and operative factors such as placement of central venous catheters and shunts. Complications of thrombosis include cerebrovascular accidents and pulmonary emboli.[42] See Figure 17.10[43] for a depiction of various causative factors of thrombosis in congenital heart disease. In this figure, CPB refers to cardiopulmonary bypass, where blood flow is routed through a machine for oxygenation and then back to the client’s circulation. CPB is used during open heart surgery when the heart is stopped.
Blood coagulation modifiers prolong the time required for blood to clot and include anticoagulants, antiplatelet agents, and thrombolytic agents. Anticoagulants, such as heparin, low-molecular weight heparin, and warfarin work directly on clotting factors. Antiplatelet agents, such as aspirin and clopidogrel, prevent platelets from clumping together and forming clots. Thrombolytic agents, such as tissue plasminogen activator (tPA), break up blood clots that have already formed. Pediatric clients are prescribed specific blood coagulation modifiers based on their specific risk factors and clinical status. Nurses closely monitor clients receiving blood coagulation modifiers for bleeding complications.[44]
Anticoagulation therapy is not generally recommended for use with pulmonary hypertension due to the increased risks of hemorrhage, hemoptysis (bloody sputum), and death.[45] The health care provider evaluates the risks and benefits, and if it is determined that anticoagulants are beneficial for a client with pulmonary hypertension, it is prescribed in a very low dose to help mitigate risks.[46]
Review information about blood coagulation modifiers in the “Blood Coagulation Modifiers” section of the “Cardiovascular & Renal Systems” chapter in Open RN Nursing Pharmacology, 2e.
Oxygen Therapy
Oxygen therapy is prescribed based on the specific type and severity of the congenital heart defect, underlying medical issues, and the client’s clinical status.
Surgical Interventions
Surgical interventions may be performed based on the specific heart defect and its severity. Common surgical interventions for cardiac defects are summarized in Table 17.5d.
Table 17.5d. Common Surgical Interventions[47],[48],[49],[50],[51],[52],[53]
| Surgical Intervention | Description | Defect Repaired |
|---|---|---|
| Heart Catheterization |
|
|
| Cardiac Surgery |
|
|
| Palliative Shunts |
|
|
| Cardiac Transplantation |
|
|
Nursing Interventions
Nursing interventions are based on the client’s specific heart defect and its severity. Selected interventions and their rationale are summarized in Table 17.5e.
Table 17.5e. Selected Nursing Interventions and Rationale[54]
| Intervention | Description | Rationale |
|---|---|---|
| Perform pulse oximetry screening on newborns |
|
|
| Strictly monitor intake and output |
|
|
| Monitor daily weights |
|
|
| Maintain stable environmental temperatures |
|
|
| Provide rest/calm environment |
|
|
| Feed infant when hungry, upon awakening, and at least every three hours |
|
|
| Safely administer digoxin and other cardiac medications as prescribed | Digoxin:
|
|
| Monitor for respiratory distress |
|
|
| Promptly intervene with hypercyanotic episodes |
|
|
| Administer oxygen as needed |
|
|
View a supplementary YouTube video[55] from American Academy of Pediatrics about critical congenital heart defect newborn screening guidelines: Critical Congenital Heart Defects: Updated Newborn Screening Guidelines for Pediatricians.
Health Teaching
Nurses provide health teaching to the parents or caregivers of infants and young children after performing a learner assessment that assesses their readiness to learn, their preferred method for learning, and their knowledge about the heart defects present and care required. Nurses are aware that diagnosis of a congenital heart defect or congenital heart disease can be devastating and highly stressful for parents, and they may have difficulty coping with a new diagnosis. Parents or caregivers will likely need simple explanations that are repeated across teaching sessions, as well as written handouts, to retain the information. Key teaching topics pertaining to CHD are described in Table 17.7f.
Table 17.7f. Key Teaching Topics Pertaining to CHD[56]
| Topic | Information |
|---|---|
| Pathophysiology of the defect |
|
| Medications |
|
| Feedings |
|
| When to call the provider |
|
Evaluation (Evaluate Outcomes)
During the evaluation stage, nurses determine the effectiveness of nursing interventions for a specific client. The previously identified expected outcomes are reviewed to determine if they were met, partially met, or not met by the time frames indicated. If outcomes are not met or only partially met by the time frame indicated, the nursing care plan is revised. Evaluation should occur every time the nurse implements interventions with a client, reviews updated laboratory or diagnostic test results, or discusses the care plan with other members of the interprofessional team.
- McKinney, E. S., James, S. R., Murray, S. S., Nelson, K., & Ashwill, J. (2018). Maternal-child nursing (5th ed.). Elsevier Saunders. ↵
- McKinney, E. S., James, S. R., Murray, S. S., Nelson, K., & Ashwill, J. (2018). Maternal-child nursing (5th ed.). Elsevier Saunders. ↵
- Ernstmeyer, K., & Christman, E. (Eds.). (2023). Nursing skills, 2e. https://wtcs.pressbooks.pub/nursingskills/ ↵
- Stanford Medicine Children's Health. (n.d.). Slow or poor infant weight gain. https://deprod.stanfordchildrens.org/en/topic/default?id=slow-or-poor-infant-weight-gain-90-P02880 ↵
- Stanford Medicine 25. (2024, May 29. Cardiac examination of the newborn [Video]. YouTube. All rights reserved. https://www.youtube.com/watch?v=8kg2FBYpn4g ↵
- Binks, A. (2024). 6.1: Congenital heart disease. LibreTexts: Medicine. https://med.libretexts.org/Courses/Virginia_Tech_Carilion_School_of_Medicine/Cardiovascular_Pathophysiology_for_Pre-Clinical_Students_(Binks)/06:_Congenital_Heart_Disease/6.01:_Congenital_heart_disease ↵
- U.S. Department of Health and Human Services. (n.d.). Congenital heart defects: Types. National Heart Lung and Blood Institute. https://www.nhlbi.nih.gov/health/congenital-heart-defects/types ↵
- Betts, J. G., Desaix, P., Johnson, E., Johnson, J. E., Korol, O., Kruse, D., Poe, B., Wise, J., Womble, M. D., & Young, K. A. (2022). Anatomy and physiology, 2e. OpenStax. https://openstax.org/details/books/anatomy-and-physiology-2e ↵
- U.S. National Library of Medicine. (2023). Cyanotic heart disease. MedlinePlus. https://medlineplus.gov/ency/article/001104.htm ↵
- Government of Western Australia. (2024). Hypercyanotic spells. Perth Children's Hospital. https://pch.health.wa.gov.au/for-health-professionals/emergency-department-guidelines/hypercyanotic-spells ↵
- McKinney, E. S., James, S. R., Murray, S. S., Nelson, K., & Ashwill, J. (2018). Maternal-child nursing (5th ed.). Elsevier Saunders. ↵
- Sharma, M., Nair, M., Jatana, S. K., & Shahi, B. N. (2003). Congestive heart failure in infants and children. Medical Journal, Armed Forces India, 59(3), 228–233. https://doi.org/10.1016/S0377-1237(03)80014-X ↵
- McKinney, E. S., James, S. R., Murray, S. S., Nelson, K., & Ashwill, J. (2018). Maternal-child nursing (5th ed.). Elsevier Saunders. ↵
- Centers for Disease Control and Prevention. (2025). Clinical screening and diagnosis for congenital heart defects. https://www.cdc.gov/heart-defects/hcp/screening/index.html ↵
- Makic, M. B. F., & Martinez-Kratz, M. R. (2023). Ackley and Ladwig's nursing diagnosis handbook: An evidence-based guide to planning care (13 ed.). Elsevier Inc. ↵
- Makic, M. B. F., & Martinez-Kratz, M. R. (2023). Ackley and Ladwig's nursing diagnosis handbook: An evidence-based guide to planning care (13 ed.). Elsevier Inc. ↵
- Surak, A. (2022). Pharmacological treatment of patent ductus arteriosus in preterm infants. IntechOpen. https://www.intechopen.com/chapters/81216 ↵
- Olgun, H., Ceviz, N., Kartal, I., Caner, I., Karacan, M., Taştekin, A., & Becit, N. (2017). Repeated courses of oral ibuprofen in premature infants with patent ductus arteriosus: Efficacy and safety. Pediatrics & Neonatology, 58(1), 29-35. https://www.sciencedirect.com/science/article/pii/S1875957216300389 ↵
- Zeller, B. (2016). The use of acetaminophen for closure of the patent ductus arteriosus. Pediatric Pharmacy Association. https://www.ppag.org/?pg=PPAGNews&blAction=showEntry&blogEntry=3572 ↵
- Cucerea, M., Simon, M., Moldovan, E., Ungureanu, M., Marian, R., & Suciu, L. (2016). Congenital heart disease requiring maintenance of ductus arteriosus in critically ill newborns admitted at a tertiary neonatal intensive care unit. Journal of Critical Care Medicine (Universitatea de Medicina si Farmacie din Targu-Mures), 2(4), 185–191. https://doi.org/10.1515/jccm-2016-0031 ↵
- Surak, A. (2022). Pharmacological treatment of patent ductus arteriosus in preterm infants. IntechOpen. https://www.intechopen.com/chapters/81216 ↵
- Olgun, H., Ceviz, N., Kartal, I., Caner, I., Karacan, M., Taştekin, A., & Becit, N. (2017). Repeated courses of oral ibuprofen in premature infants with patent ductus arteriosus: Efficacy and safety. Pediatrics & Neonatology, 58(1), 29-35. https://www.sciencedirect.com/science/article/pii/S1875957216300389 ↵
- Zeller, B. (2016). The use of acetaminophen for closure of the patent ductus arteriosus. Pediatric Pharmacy Association. https://www.ppag.org/?pg=PPAGNews&blAction=showEntry&blogEntry=3572 ↵
- David, M. N. V., & Shetty, M. (2024). Digoxin. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK556025/ ↵
- Sharma, M., Nair, M., Jatana, S. K., & Shahi, B. N. (2003). Congestive heart failure in infants and children. Medical Journal, Armed Forces India, 59(3), 228–233. https://doi.org/10.1016/S0377-1237(03)80014-X ↵
- Sharma, M., Nair, M., Jatana, S. K., & Shahi, B. N. (2003). Congestive heart failure in infants and children. Medical Journal, Armed Forces India, 59(3), 228–233. https://doi.org/10.1016/S0377-1237(03)80014-X ↵
- David, M. N. V., & Shetty, M. (2024). Digoxin. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK556025/ ↵
- McKinney, E. S., James, S. R., Murray, S. S., Nelson, K., & Ashwill, J. (2018). Maternal-child nursing (5th ed.). Elsevier Saunders. ↵
- Sharma, M., Nair, M., Jatana, S. K., & Shahi, B. N. (2003). Congestive heart failure in infants and children. Medical Journal, Armed Forces India, 59(3), 228–233. https://doi.org/10.1016/S0377-1237(03)80014-X ↵
- Ernstmeyer, K., & Christman, E. (2023). Nursing pharmacology, 2e. Open RN. https://wtcs.pressbooks.pub/pharmacology2e/ ↵
- Doshi, U., & Wang-Giuffre, E. (2022). Ventricular septal defects: A review. IntechOpen. https://www.intechopen.com/chapters/81658 ↵
- Cooper, D., & Dimri, M. (2023). Calcium channels. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK562198/ ↵
- Pérez-Serra, A., Toro, R., Sarquella-Brugada, G., de Gonzalo-Calvo, D., Cesar, S., Carro, E., Llorente-Cortes, V., Iglesias, A., Brugada, J., Brugada, R., & Campuzano, O. (2016). Genetic basis of dilated cardiomyopathy. International Journal of Cardiology, 224, 461-472. https://doi.org/10.1016/j.ijcard.2016.09.068 ↵
- Cooper, D., & Dimri, M. (2023). Calcium channels. StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK562198/ ↵
- Stone, M. L., Kelly, J., Mistry, M., Buck, M., Gangemi, J., & Vergales, J. (2018). Use of nicardipine after cardiac operations is safe in children regardless of age. The Annals of Thoracic Surgery, 105(1), 181–185. https://doi.org/10.1016/j.athoracsur.2017.05.035 ↵
- Sharma, M., Nair, M., Jatana, S. K., & Shahi, B. N. (2003). Congestive heart failure in infants and children. Medical Journal, Armed Forces India, 59(3), 228–233. https://doi.org/10.1016/S0377-1237(03)80014-X ↵
- Pascall, E., & Tulloh, R. M. (2018). Pulmonary hypertension in congenital heart disease. Future Cardiology, 14(4), 343–353. https://doi.org/10.2217/fca-2017-0065 ↵
- American Heart Association. (2023). Pulmonary hypertension. Circulation: Heart failure. 16(7). https://doi.org/10.1161/HHF.0000000000000080. ↵
- Steinhorn, R. H. (2010). Neonatal pulmonary hypertension. Pediatric Critical Care Medicine: A Journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies, 11(2 Suppl), S79–S84. https://doi.org/10.1097/PCC.0b013e3181c76cdc ↵
- Steinhorn. R. H. (2010). Neonatal pulmonary hypertension. Pediatric Critical Care Medicine: A Journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies, 11(2 Suppl), S79–S84. https://doi.org/10.1097/PCC.0b013e3181c76cdc ↵
- Merck Manual Consumer Edition. (2023). Persistent pulmonary hypertension of the newborn. Merck Manuals. https://www.merckmanuals.com/home/children-s-health-issues/lung-and-breathing-problems-in-newborns/persistent-pulmonary-hypertension-of-the-newborn ↵
- Abdelghani, E., Cua, C. L., Giver, J., & Rodriguez, V., (2021). Thrombosis prevention and anticoagulation management in the pediatric patient with congenital heart disease. Cardiology and Therapy, 10(2), 325–348. https://doi.org/10.1007/s40119-021-00228-4 ↵
- “40119_2021_228_Fig1_HTML” by Eman Abdelghai, Clifford L Cua, Jean Giver, & Vilmari Rodriguez is licensed under CC BY-NC 4.0 ↵
- Abdelghani, E., Cua, C. L., Giver, J., & Rodriguez, V., (2021). Thrombosis prevention and anticoagulation management in the pediatric patient with congenital heart disease. Cardiology and Therapy, 10(2), 325–348. https://doi.org/10.1007/s40119-021-00228-4 ↵
- Pascall, E., & Tulloh, R. M. (2018). Pulmonary hypertension in congenital heart disease. Future Cardiology, 14(4), 343–353. https://doi.org/10.2217/fca-2017-0065 ↵
- Abdelghani, E., Cua, C. L., Giver, J., & Rodriguez, V., (2021). Thrombosis prevention and anticoagulation management in the pediatric patient with congenital heart disease. Cardiology and Therapy, 10(2), 325–348. https://doi.org/10.1007/s40119-021-00228-4 ↵
- American Heart Association. (2023). Cardiac catheterizations for heart defects. https://www.heart.org/en/health-topics/congenital-heart-defects/care-and-treatment-for-congenital-heart-defects/cardiac-catheterizations-for-heart-defects ↵
- American Heart Association. (2023). Heart transplant. https://www.heart.org/en/health-topics/congenital-heart-defects/care-and-treatment-for-congenital-heart-defects/heart-transplant ↵
- U.S. National Library of Medicine. (2023). Congenital heart defect - corrective surgery. MedlinePlus. https://medlineplus.gov/ency/article/002948.htm ↵
- Cleveland Clinic. (2022). Cardiopulmonary bypass. https://my.clevelandclinic.org/health/treatments/24106-cardiopulmonary-bypass ↵
- Backer, C. L., & Mavroudis, C. (2023). Palliative operations. Pediatric Cardiac Surgery, 143–159. https://doi.org/10.1002/9781119282327.ch8. https://onlinelibrary.wiley.com/doi/epdf/10.1002/9781119282327.ch8 ↵
- Yuan, S.-M., & Jing, H. (2009). Palliative procedures for congenital heart defects. Archives of Cardiovascular Diseases, 102(6–7), 549-557. https://doi.org/10.1016/j.acvd.2009.04.011. https://www.sciencedirect.com/science/article/pii/S1875213609001697 ↵
- Donné, M., De Pauw, M., Vandekerckhove, K., Bové, T., & Panzer, J. (2021). Ethical and practical dilemmas in cardiac transplantation in infants: a literature review. European Journal of Pediatrics, 180(8), 2359–2365. https://doi.org/10.1007/s00431-021-04100-4 ↵
- McKinney, E. S., James, S. R., Murray, S. S., Nelson, K., & Ashwill, J. (2018). Maternal-child nursing (5th ed.). Elsevier Saunders. ↵
- American Academy of Pediatrics. (2024, December 16). Critical congenital heart defects: Updated newborn screening guidelines for pediatricians [Video]. YouTube. All rights reserved. https://www.youtube.com/watch?v=QziC0O0TGxI ↵
- McKinney, E. S., James, S. R., Murray, S. S., Nelson, K., & Ashwill, J. (2018). Maternal-child nursing (5th ed.). Elsevier Saunders. ↵
Improvement in cardiac output by increased contractility (force of the cardiac contraction).
A decrease in contractility (force of cardiac contraction), which results in decreased cardiac output.
A procedure of providing an initial loading dose of digoxin over 24 hours to rapidly raise serum digoxin levels.
A condition caused by excessive levels of digoxin, a cardiac glycoside used to treat heart failure and atrial fibrillation.
The part of the muscle responsible for releasing and storing calcium ions in muscle fibers, causing contraction and relaxation of muscles.
Blood clots.
Bloody sputum.
A procedure where a balloon catheter is inserted in a narrowed blood vessel and is inflated to widen the vessel.
A procedure where a balloon catheter is inserted into a narrowed (stenosed) valve and inflated to widen the valve.
A medical device that routes a client’s blood around the heart and lungs and oxygenates it. Cannulas (special large tubes) are placed into the vena cava where oxygen-poor blood from the body moves into the bypass machine and is oxygenated. From the bypass machine, the oxygenated blood is moved through a cannula into the aorta, where it is pushed out to the body.

