17.3 Categories of Congenital Heart Defects – Acyanotic and Cyanotic Defects
Congenital heart defects (CHDs) are structural abnormalities in the heart or blood vessels that are present at birth and affect how blood flows through the heart and out to the rest of the body. Heart defects can vary from mild (such as a small hole in the heart) to severe (such as missing parts of the heart). In the United States, heart defects affect 1% of births annually, or about 40,000 infants. Of those infants born with a heart defect, about 25% have a severe heart defect requiring surgery or other procedures in the first year of life.[1]
CHDs are present in approximately 1% of births, and although the cause is not clearly understood, they have been linked to maternal hyperglycemia, smoking during pregnancy, and certain medications taken during the first trimester of pregnancy. Some CHDs may be detected during prenatal screening, and others may be detected shortly after birth through the use of pulse oximetry in newborn screenings. Other CHDs that are asymptomatic may be detected incidentally during diagnostic testing for other conditions.[2]
Congenital heart defects are classified as either cyanotic defects that cause hypoxia due to shunting of blood from the right side to the left side of the heart, or acyanotic defects that affect the flow of blood through the heart and may affect oxygenation due to pulmonary congestion. Infants with cyanotic heart defects are sometimes referred to as “blue babies,” and infants with acyanotic heart defects are sometimes referred to as “pink babies” because of the chronic effect of the defect on their oxygenation status. An overview of common types of congenital defects is discussed in the following subsections based on these classifications.[3] Specific assessments, diagnostic tests, and treatments for congenital heart defects are further described in the “Applying the Nursing Process and Clinical Judgment Model to Congenital Heart Defects” section of this chapter.
Acyanotic Defects
Acyanotic defects affect the normal blood flow through the heart when there is an abnormal opening in the heart that causes blood to be shunted from the left side of the heart back to the right side of the heart. Systemic vascular resistance is normally higher on the left side of the heart than on the right side, and blood follows the path of least resistance, so if there is an abnormal opening between the left and right sides of the heart, blood will be shunted from left to right through the opening. Because blood on the left side of the heart has already passed through the lungs and is oxygenated, blood that is shunted is oxygen-rich and does not cause hypoxia or cyanosis. However, the shunting from the left side of the heart to the right side causes increased fluid volume in the right side of the heart. If left untreated, this increased fluid volume can lead to right-sided heart failure with blood backing up into the body and the peripheral vascular system. Common types of acyanotic congenital heart defects include patent ductus arteriosus, atrial septal defects, and ventricular septal defects.[4],[5]
Patent Ductus Arteriosus
A patent ductus arteriosus (PDA) occurs when the ductus arteriosus does not close after birth. See Figure 17.3[6] for an illustration comparing a normal heart to a heart with patent ductus arteriosus. Recall that the ductus arteriosus is a normal shunt in fetal circulation to connect the pulmonary trunk to the aorta, causing most of the circulating oxygenated blood to bypass the fetal lungs. As the newborn takes its first breaths and the lungs expand, the vascular resistance and lung pressure abruptly decrease, causing the ductus arteriosus to constrict and close. However, when a PDA is present, the opening continues to exist between the aorta and the pulmonary artery, causing oxygen-rich blood from the aorta to flow backwards into the pulmonary artery. This backwards flow can cause increased fluid volume in the right side of the heart and lungs. If left untreated, it can result in pulmonary congestion and pulmonary edema, pulmonary hypertension, and decreased blood flow out the aorta to the body.[7],[8]
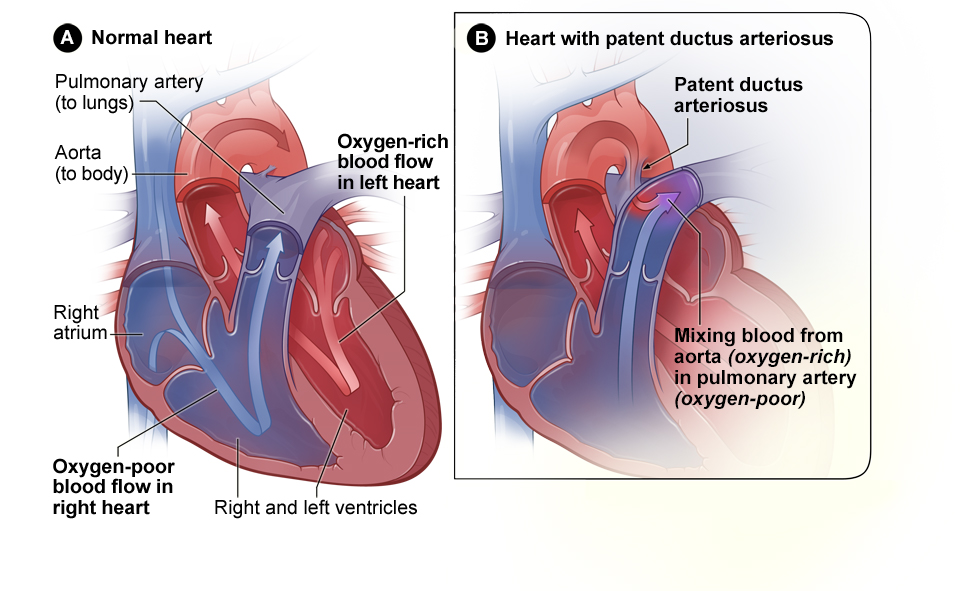
Small PDAs often close on their own and are asymptomatic. Larger PDAs are often symptomatic and require treatment. Symptoms of PDA include tachypnea, dyspnea (shortness of breath), difficulty feeding and poor weight gain in infants, tachycardia, a continuous machine-like heart murmur, an enlarged heart, and widened pulse pressure (an increased difference between systolic and diastolic blood pressure). Medical treatment may include nonsteroidal anti-inflammatory drugs (NSAIDs) to block the synthesis of prostaglandin E2 that maintains the ductus arteriosus in an open position. Surgical treatments include ligation (tying off the opening) or manual closure using platinum coils or specialized mesh inserted via the femoral artery or vein.[9],[10]
Atrial Septal Defect
Atrial septal defects (ASD) occur when there is a hole in the septum that separates the right and left atria of the heart. This defect allows blood to shunt from the left atrium to the right atrium rather than moving from the left atrium to the left ventricle to be pumped out to the body. As a result, there is an increased blood volume in the right side of the heart, which can cause blood to back up into the peripheral vascular system. ASDs often close on their own during childhood as the heart grows.[11] See Figure 17.4[12] for an illustration comparing the cross-section of a normal heart (A) and a heart with an atrial septal defect (B).
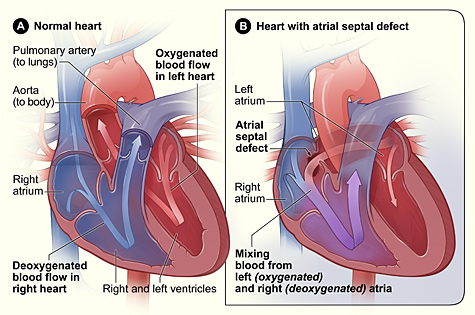
A patent foramen ovale (PFO) is an opening between the right and left atria that is a normal part of fetal circulation but fails to close at birth when the infant takes its first breaths and pressures within the atria begin to rise. Similarly to an atrial septal defect, a PFO also allows blood to flow from the left atrium to the right atrium. Small PFOs are typically asymptomatic. As many as 15-25% adults have PFOs that are found incidentally on diagnostic tests for other conditions or on autopsy.[13],[14]
ASDs and PFOs are typically detected by auscultation of a heart murmur, a blowing or whooshing sound that signifies turbulent blood flow. The diagnosis is confirmed through diagnostic imaging with an echocardiogram. Benign (asymptomatic) defects are generally monitored while symptomatic defects affecting oxygenation status typically require surgical repair.[15]
Ventricular Septal Defect
Ventricular septal defects (VSD) occur when there is a hole in the septum separating the right and left ventricles. This defect allows blood to shunt from the left ventricle, where there is higher pressure, to the right ventricle, where there is lower pressure, rather than solely being pumped through the aorta to the rest of the body. This results in blood going backward to the right ventricle to the lungs, where it is pumped again to the left atrium and left ventricle, causing the heart to work harder to pump blood. This loop continues and can cause increased fluid volume and increased pressure in the right side of the heart and lungs, contributing to the development of pulmonary congestion, pulmonary edema, pulmonary hypertension, or the development of right-sided heart failure over time.[16],[17] See Figure 17.5 for an illustration comparing a normal heart and a heart with a ventricular septal defect.
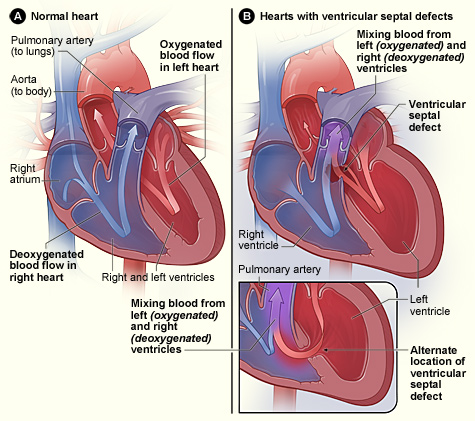
VSDs are commonly detected through auscultation of a murmur due to turbulent blood flow through the ventricular septum and are diagnosed by an echocardiogram. In many cases, small VSDs may close on their own as the heart grows, or they may be asymptomatic and not require treatment. Moderate to large VSDs often require surgical repair to treat or prevent right-sided heart failure or pulmonary complications.[18],[19],[20]
Cyanotic Defects
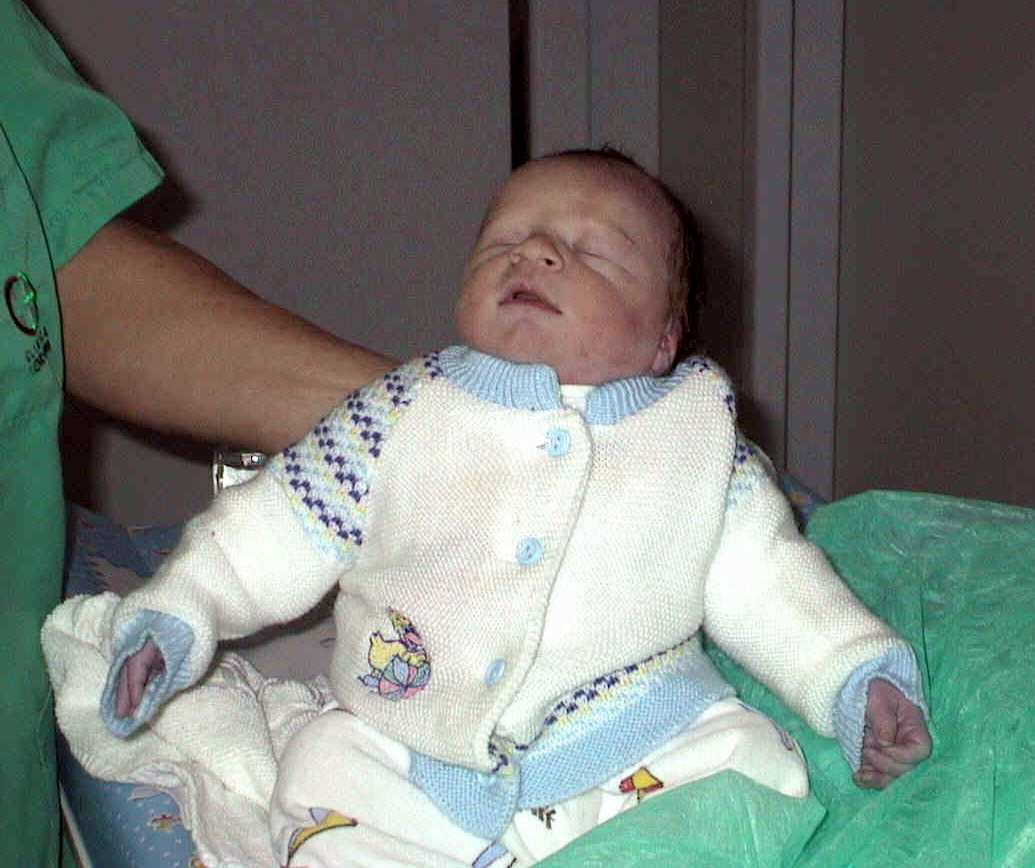
Cyanotic heart defects involve right to left shunting of blood through the heart, which causes deoxygenated blood from the venous system to bypass transport to the lungs and is pumped through the aorta to the arterial system. Recall that in a normal heart, blood flows from the right ventricle to the lungs where it is oxygenated and then returns back to the left atrium and left ventricle where it is pumped through the aorta to the rest of the body. When a cyanotic defect is present, poorly oxygenated blood is pumped to the body, causing decreased blood oxygen saturation levels, organ and tissue hypoxia, and cyanosis. Because the blood bypasses the lungs, oxygen administration does little to improve oxygen saturation levels. Cyanosis manifests as a bluish color to the lips, fingers, and toes of an infant or child and may occur at rest or with activity, depending on the severity of the heart defect.[21] As such, the term “blue baby” has been used to describe children with cyanotic heart defects. See Figure 17.6[22] for a depiction of a “blue baby” with cyanosis associated with cyanotic congenital heart defects.
Chronic hypoxia seen with cyanotic defects can result in polycythemia (i.e., elevated number of red blood cells). Excess red blood cells are produced as a compensatory mechanism by the body to attempt to increase the oxygen-carrying capacity of the blood. However, despite increased numbers of red blood cells, a person with a cyanotic heart defect still remains cyanotic unless the specific defect is corrected. Polycythemia can be problematic because the blood becomes more viscous (thicker). A client with increased viscosity is at risk for thrombosis (clotting) because blood flows sluggishly, giving time for clots to develop that can embolize and cause a cerebrovascular accident (CVA) or pulmonary emboli. Additionally, increased red blood cells crowd the intravascular space, resulting in less room for plasma and clotting factors, placing the client at risk for bleeding when there are reduced clotting factors in proportion to blood volume. Anemia may also develop when there is not enough iron to produce sufficient hemoglobin for the increased number of red blood cells.[23]
Sometimes infants and children with cyanotic heart defects may experience hypercyanotic episodes. These episodes are common with clients with Tetralogy of Fallot and are commonly called “Tet spells.” Tetralogy of Fallot is further discussed in the following subsection. During a hypercyanotic episode, there is a sudden increase in shunting of blood from the right to left side of the heart, resulting in severely decreased blood flow through the lungs and acute hypoxia in the tissues of the body. A hypercyanotic episode can be caused by a variety of stimuli such as stress, straining to have a bowel movement, exertion through extreme crying or feeding sessions, fever, dehydration, or even just awakening from a nap. Signs of hypercyanotic episodes typically include a period of irritability or uncontrollable crying, hyperventilation, and signs of increasing hypoxia with cyanosis. The infant may become limp and lose consciousness, and untreated episodes can result in death.[24]
Treatment for hypercyanotic episodes includes trying to calm the infant while placing them in a position with their knees to their chest. Older children will instinctively squat to bring their knees to their chest and should not be stopped from assuming this position. This knee-to-chest position increases systemic vascular resistance, thereby increasing pressure in the left side of the heart and reducing blood shunting from the right side to the left side of the heart, resulting in more blood being transported to the lungs for oxygenation. During a hypercyanotic episode, 100% oxygen should be administered to increase the oxygenation saturation of the blood that is being transported through the lungs. However, if oxygen administration through a face mask causes distress in the infant or child, it should be applied by blow-by method. Blow-by method entails directing a flow of oxygen at high concentration near the client’s face without making direct contact. Morphine administration may be prescribed to decrease respiration rate and heart rate, which decreases oxygen demand and improves blood flow to the lungs. Advanced life support measures may be required if a hypercyanotic episode is not reversed.[25]
Tetralogy of Fallot
Tetralogy of Fallot (ToF) is a complex set of congenital heart defects. The term tetralogy is derived from the four components associated with the condition, although only three may be present to be diagnosed with the disorder. The four components include pulmonary infundibular stenosis (rigidity or stenosis of the pulmonary valve), a large ventricular septal defect (opening between the ventricles), an overriding aorta (the aorta is shifted above both ventricles and centered over the ventricular septal defect), and right ventricular hypertrophy (enlargement of the right ventricle). Other heart defects may also accompany this condition. A heart with Tetralogy of Fallot often has a “boot-like” appearance when viewed on chest X-ray. See Figure 17.7[26] for an illustration comparing a normal heart and a heart with Tetralogy of Fallot. Note that in this image, the purple arrow indicates mixed blood coming from both the right and left side of the heart.[27]
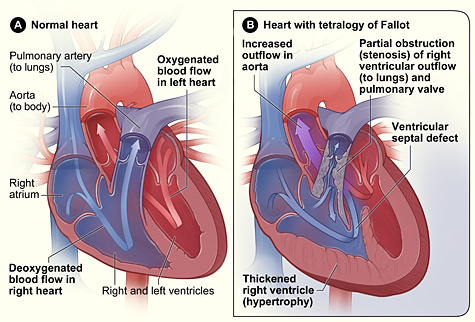
The components of ToF cause multiple effects. Pulmonary stenosis makes it difficult for blood to flow from the right ventricle into the lungs, causing increased pressure inside the right ventricle. This causes the right ventricle to work harder to pump blood, causing right ventricular hypertrophy that can lead to heart failure. It also causes deoxygenated blood from the right ventricle to move through the ventricular septal defect into the left ventricle. Because the aorta is located between the ventricles and over the ventricular septal defect, oxygenated blood from the left atrium mixes with deoxygenated blood from the right ventricle, and this partially oxygenated blood moves through the aorta and to the rest of the body, causing hypoxia and cyanosis.[28]
Signs of ToF include a distinct heart murmur, low blood oxygen saturation levels (SpO2), dyspnea, polycythemia, clubbing (broadening) of the fingers and toes, difficulty feeding, and failure to grow. A pulse oximetry screening tool is used in hospitals after childbirth to detect signs of Critical Congenital Heart Defects (CCHD) such as ToF. During this screening test, pulse oximetry is performed at 24 hours of age on the right hand (preductal) and either foot (postductal). If both readings are greater or equal to 95% and there is no more than a 3% difference between the two readings, the infant is at less risk for ToF. Other cardiac conditions that can be detected with this screening tool include coarctation of the aorta, hypoplastic left heart syndrome, pulmonary atresia, d-Transposition of the great arteries, and persistent pulmonary hypertension.[29],[30]
ToF is diagnosed by echocardiography. Treatment involves extensive surgical repair, including stents to redirect blood flow, valve replacement, and patches to repair the septal defect.[31] Surgical repair results in excellent long-term survival in the United States, with over 90% survival rates for individuals twenty-five years after repair. However, heart failure and arrhythmias are common long-term consequences that can lead to death.[32]
Transposition of the Great Arteries (TGA)
Transposition of the great arteries (TGA) is a congenital heart defect in which the positions of the pulmonary artery and the aorta are switched, resulting in abnormal circulation of blood. In normal cardiac anatomy, the pulmonary artery carries deoxygenated blood from the right ventricle to the lungs, and the aorta carries oxygenated blood from the left ventricle to the rest of the body. However, in transposition of the great arteries, the aorta arises from the right ventricle and the pulmonary artery arises from the left ventricle. This abnormal configuration causes deoxygenated blood to be pumped to the body, while oxygenated blood is sent back to the lungs, where it cannot deliver oxygen to the tissues. As a result, systemic oxygenation is severely diminished. Causes of TGA are unknown but the risk increases in families with a history of congenital heart defects, and pregnancy related health concerns such as smoking, drinking, and infections contracted during pregnancy.[33]
In severe cases, this condition is not compatible with life unless there is an additional abnormality, such as a patent foramen ovale or a ventricular septal defect, which allows for mixing of oxygenated and deoxygenated blood. Without intervention, infants with TGA typically present with cyanosis (a bluish tint to the skin due to low oxygen levels), respiratory distress, and circulatory failure. Treatment often involves surgical intervention, such as an arterial switch operation, to correct the positioning of the great vessels and restore normal circulation.[34]
Hypoplastic Left Heart Syndrome
Hypoplastic left heart syndrome (HLHS) is a congenital heart defect characterized by underdevelopment of the left side of the heart, including the left ventricle, left atrium, mitral valve, and aortic valve. In a normally functioning heart, the left side is responsible for pumping oxygenated blood to the body. However, in HLHS, the left side of the heart is too small and too weak to perform this function effectively, resulting in severe deficit of oxygenated blood throughout the body. Typically, the right side of the heart compensates for the weakened left side by pumping blood into the lungs and the rest of the body through the patent ductus arteriosus (PDA) and the foramen ovale, which are two fetal structures that remain open temporarily after birth. However, this can only sustain life for a short period of time. As the ductus arteriosus closes naturally in the first few days of life, the lack of a functional left ventricle causes blood flow to be disrupted, leading to circulatory collapse. The infant may exhibit symptoms such as severe cyanosis (a bluish tint to the skin), difficulty breathing, and shock.[35]
The exact cause of HLHS is not known, but it is thought to result from a combination of genetic and environmental factors, including maternal health issues, such as diabetes or certain medications during pregnancy. Family history of congenital heart defects also increases the risk of HLHS.[36]
Due to the critical nature of HLHS, treatment typically involves a series of surgeries starting shortly after birth to establish a functional circulation. The surgeries are typically performed in a series of three-staged surgeries: the Norwood procedure (Stage 1), performed within the first week of life, creates a functional aorta and uses the right ventricle to pump blood to the body; the Glenn procedure (Stage 2), done at 3-6 months, redirects blood from the upper body directly to the lungs, reducing the right ventricle’s workload; and the Fontan procedure (Stage 3), performed at 2-4 years, completes the transition to single-ventricle circulation by rerouting the inferior vena cava to the lungs. These surgeries allow the right ventricle to manage both systemic and pulmonary circulation, though long-term care is needed to monitor heart function, as the right ventricle remains under strain.[37]
The goal is to reroute blood flow so that the right side of the heart can take over the function of the left side. Although HLHS is a serious and life-threatening condition, many children with HLHS go on to lead active lives, though ongoing medical care is necessary.[38]
Left-Sided Obstructive Defects
Left-sided obstructive defects are also considered cyanotic heart defects, but their pathophysiology is different from ToF. Rather than causing blood to shunt from the right to the left side of the heart, obstructive defects cause a restriction, or obstruction, in blood flow being pumped from the left side of the heart. These defects may be caused by lesions, stenosis, or mechanical obstructions. Left-sided obstructive defects result in the heart pumping harder to eject blood and can lead to fluid and pressure overload in the lungs and left side of the heart, contributing to pulmonary hypertension and left-sided heart failure. They can also cause increased pressures in the top half of the body and decreased pressures in the lower half of the body. Examples of left-sided obstructive defects include coarctation of the aorta and congenital mitral valve stenosis. These defects will be discussed in the following subsections.[39]
Coarctation of the Aorta
Coarctation of the aorta, or critical aortic stenosis, is an abnormal narrowing, or constriction, of the aorta. This narrowing is usually located at or near the ligamentum arteriosum, where the fetal ductus arteriosus is located prior to its closure after birth. See Figure 17.8[40] for an illustration of coarctation of the aorta that is delineated with a circle. If severe, this condition drastically restricts blood flow through the aorta, resulting in poor blood flow to the lower half of the body. Blood flow through the aorta to the top half of the body is generally intact because the narrowing usually occurs after the carotid and subclavian arteries. This can result in increased blood pressure readings in the upper extremities and decreased blood pressure in the lower extremities. Decreased blood pressure in the lower half of the body can also impact perfusion of the kidneys, resulting in decreased urine output. As a result of the narrowing, the heart pumps harder, which can cause ventricular hypertrophy. Backup of blood in the left side of the heart to the lungs can lead to left-sided heart failure and pulmonary hypertension.[41]
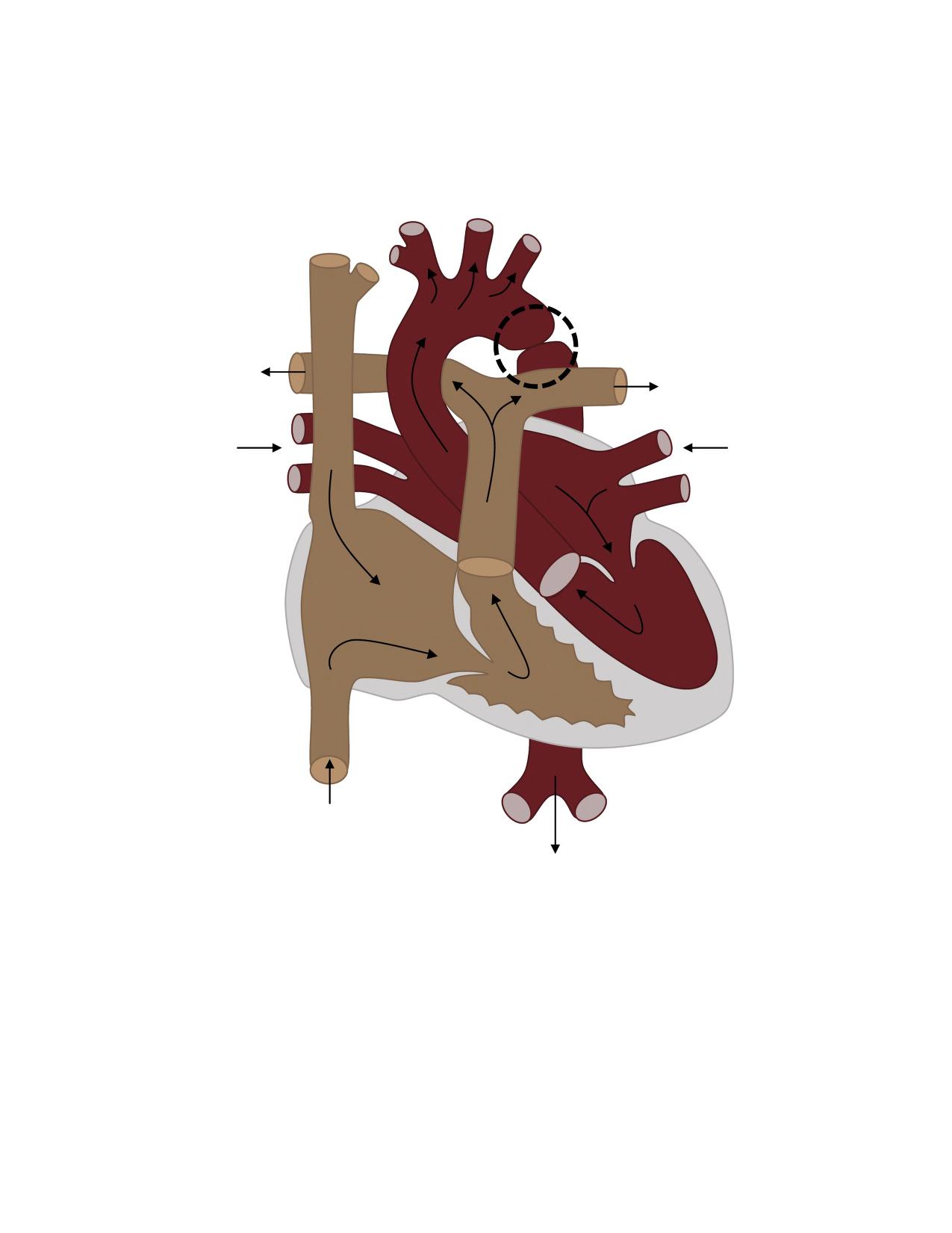
In some individuals, coarctation of the aorta may be asymptomatic and not detected until later in life. Detectable symptoms in an infant include increased work of breathing, poor appetite, trouble feeding, or failure to thrive. In older individuals, symptoms include dizziness, fainting, shortness of breath, chest pain, fatigue, headache, and nosebleeds. Treatment involves surgery to resect (remove) the narrowed area or angioplasty to open the narrow passageway. Studies have shown that the earlier the surgery is performed, the better the chance of survival.[42]
Congenital Mitral Stenosis
Congenital mitral stenosis is a narrowing of the mitral valve, located between the left atrium and left ventricle that is present at birth. See Figure 17.9[43] for an illustration of a heart with mitral stenosis. The extent of tissues involved in the valve can vary. The stenosis may be caused by an anatomical obstruction, such as extra tissue blocking the mitral valve, or a functional obstruction, such as malformed valve leaflets, missing chordae tendineae, or dysfunctional papillary muscles. Both anatomical and functional obstructions prevent the valve from working as it should, resulting in blood backing up into the left atrium and into pulmonary vasculature, with less blood available to pump through the left ventricle to the rest of the body. Symptoms depend on the severity of the stenosis and decreased cardiac output and may include dyspnea, poor appetite, trouble feeding, or failure to thrive. Over time severe stenosis can cause left-sided heart failure or pulmonary hypertension.[44],[45]
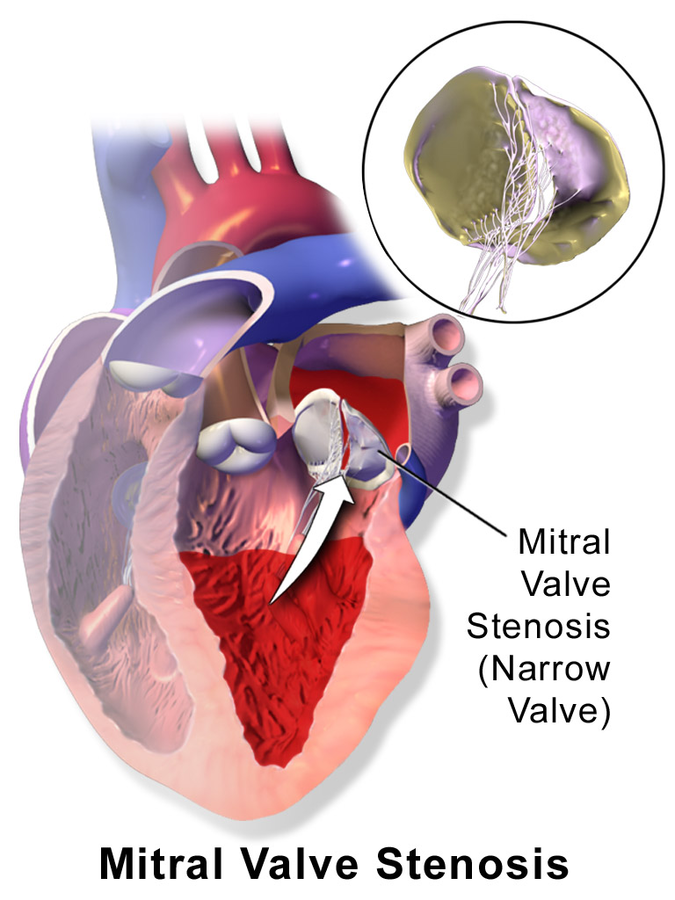
Congenital mitral stenosis is generally suspected with auscultation of a murmur over the mitral valve. An echocardiogram confirms the diagnosis. For asymptomatic or mild-to-moderate stenosis, routine monitoring with regular physical examinations and echocardiograms are sufficient to monitor the stability of the mitral valve. Symptomatic mitral stenosis may require antihypertensive and dysrhythmic medications to control blood pressure, heart rhythm, and heart function. The client may also need to take prophylactic antibiotics prior to medical or dental procedures to prevent bacterial endocarditis. In severe cases, surgical intervention may be required, such as balloon valvuloplasty, valve repair, or valve replacement.[46]
- Centers for Disease Control and Prevention. (n.d.). About congenital heart defects. https://www.cdc.gov/heart-defects/about/index.html ↵
- Centers for Disease Control and Prevention. (n.d.). About congenital heart defects. https://www.cdc.gov/heart-defects/about/index.html ↵
- Centers for Disease Control and Prevention. (n.d.). About congenital heart defects. https://www.cdc.gov/heart-defects/about/index.html ↵
- Binks, A. (2024). 6.1: Congenital heart disease. LibreTexts: Medicine. https://med.libretexts.org/Courses/Virginia_Tech_Carilion_School_of_Medicine/Cardiovascular_Pathophysiology_for_Pre-Clinical_Students_(Binks)/06:_Congenital_Heart_Disease/6.01:_Congenital_heart_disease ↵
- U.S. Department of Health and Human Services. (n.d.). Congenital heart defects: Types. National Heart Lung and Blood Institute. https://www.nhlbi.nih.gov/health/congenital-heart-defects/types ↵
- “patent_ductus_arteriosus” by unknown author, National Heart, Lung, and Blood Institute is in the Public Domain. Access for free at https://www.nhlbi.nih.gov/health/congenital-heart-defects/types ↵
- Binks, A. (2024). 6.1: Congenital heart disease. LibreTexts: Medicine. https://med.libretexts.org/Courses/Virginia_Tech_Carilion_School_of_Medicine/Cardiovascular_Pathophysiology_for_Pre-Clinical_Students_(Binks)/06:_Congenital_Heart_Disease/6.01:_Congenital_heart_disease ↵
- U.S. Department of Health and Human Services. (n.d.). Congenital heart defects: Types. National Heart Lung and Blood Institute. https://www.nhlbi.nih.gov/health/congenital-heart-defects/types ↵
- Binks, A. (2024). 6.1: Congenital heart disease. LibreTexts: Medicine. https://med.libretexts.org/Courses/Virginia_Tech_Carilion_School_of_Medicine/Cardiovascular_Pathophysiology_for_Pre-Clinical_Students_(Binks)/06:_Congenital_Heart_Disease/6.01:_Congenital_heart_disease ↵
- Betts, J. G., Desaix, P., Johnson, E., Johnson, J. E., Korol, O., Kruse, D., Poe, B., Wise, J., Womble, M. D., & Young, K. A. (2022). Anatomy and physiology, 2e. OpenStax. https://openstax.org/details/books/anatomy-and-physiology-2e ↵
- U.S. Department of Health and Human Services. (n.d.). Congenital heart defects: Types. National Heart Lung and Blood Institute. https://www.nhlbi.nih.gov/health/congenital-heart-defects/types ↵
- “atrial_septal_defect” by unknown author, National Heart, Lung, and Blood Institute is in the Public Domain. Access for free at https://www.nhlbi.nih.gov/health/congenital-heart-defects/types ↵
- Medstar Health. (2025). Patent foramen ovales and atrial septal defects. medstarthealth.org. https://www.medstarhealth.org/services/patent-foramen-ovale-atrial-septal-defects-treatments ↵
- McCabe, L. (2020). Fetal circulation [Video]. YouTube. OpenPediatrics. https://youtu.be/HVBu9HhTkD4?feature=shared ↵
- Betts, J. G., Desaix, P., Johnson, E., Johnson, J. E., Korol, O., Kruse, D., Poe, B., Wise, J., Womble, M. D., & Young, K. A. (2022). Anatomy and physiology, 2e. OpenStax. https://openstax.org/details/books/anatomy-and-physiology-2e ↵
- Binks, A. (2024). 6.1: Congenital heart disease. LibreTexts: Medicine. https://med.libretexts.org/Courses/Virginia_Tech_Carilion_School_of_Medicine/Cardiovascular_Pathophysiology_for_Pre-Clinical_Students_(Binks)/06:_Congenital_Heart_Disease/6.01:_Congenital_heart_disease ↵
- U.S. Department of Health and Human Services. (n.d.). Congenital heart defects: Types. National Heart Lung and Blood Institute. https://www.nhlbi.nih.gov/health/congenital-heart-defects/types ↵
- Binks, A. (2024). 6.1: Congenital heart disease. LibreTexts: Medicine. https://med.libretexts.org/Courses/Virginia_Tech_Carilion_School_of_Medicine/Cardiovascular_Pathophysiology_for_Pre-Clinical_Students_(Binks)/06:_Congenital_Heart_Disease/6.01:_Congenital_heart_disease ↵
- Betts, J. G., Desaix, P., Johnson, E., Johnson, J. E., Korol, O., Kruse, D., Poe, B., Wise, J., Womble, M. D., & Young, K. A. (2022). Anatomy and physiology, 2e. OpenStax. https://openstax.org/details/books/anatomy-and-physiology-2e ↵
- U.S. Department of Health and Human Services. (n.d.). Congenital heart defects: Types. National Heart Lung and Blood Institute. https://www.nhlbi.nih.gov/health/congenital-heart-defects/types ↵
- U.S. National Library of Medicine. (2023). Cyanotic heart disease. MedlinePlus. https://medlineplus.gov/ency/article/001104.htm ↵
- “HLHS” by Cornelia Csuk is licensed under CC BY-SA 3.0 ↵
- McKinney, E. S., James, S. R., Murray, S. S., Nelson, K., & Ashwill, J. (2018). Maternal-child nursing (5th ed.). Elsevier Saunders. ↵
- Betts, J. G., Desaix, P., Johnson, E., Johnson, J. E., Korol, O., Kruse, D., Poe, B., Wise, J., Womble, M. D., & Young, K. A. (2022). Anatomy and physiology, 2e. OpenStax. https://openstax.org/details/books/anatomy-and-physiology-2e ↵
- Government of Western Australia. (2024). Hypercyanotic spells. Perth Children's Hospital. https://pch.health.wa.gov.au/for-health-professionals/emergency-department-guidelines/hypercyanotic-spells ↵
- “tetralogy_fallot” by unknown author, National Heart, Lung, and Blood Institute is in the Public Domain. Access for free at https://www.nhlbi.nih.gov/health/congenital-heart-defects/types ↵
- Betts, J. G., Desaix, P., Johnson, E., Johnson, J. E., Korol, O., Kruse, D., Poe, B., Wise, J., Womble, M. D., & Young, K. A. (2022). Anatomy and physiology, 2e. OpenStax. https://openstax.org/details/books/anatomy-and-physiology-2e ↵
- Betts, J. G., Desaix, P., Johnson, E., Johnson, J. E., Korol, O., Kruse, D., Poe, B., Wise, J., Womble, M. D., & Young, K. A. (2022). Anatomy and physiology, 2e. OpenStax. https://openstax.org/details/books/anatomy-and-physiology-2e ↵
- Centers for Disease Control and Prevention. (2024). Screening for critical congenital heart defects. https://www.cdc.gov/heart-defects/screening/index.html ↵
- Betts, J. G., Desaix, P., Johnson, E., Johnson, J. E., Korol, O., Kruse, D., Poe, B., Wise, J., Womble, M. D., & Young, K. A. (2022). Anatomy and physiology, 2e. OpenStax. https://openstax.org/details/books/anatomy-and-physiology-2e ↵
- Betts, J. G., Desaix, P., Johnson, E., Johnson, J. E., Korol, O., Kruse, D., Poe, B., Wise, J., Womble, M. D., & Young, K. A. (2022). Anatomy and physiology, 2e. OpenStax. https://openstax.org/details/books/anatomy-and-physiology-2e ↵
- Doyle, T., & Kavanaugh-McHugh, A. (2023). Tetralogy of Fallot (TOF): Management and outcome. UpToDate. https://www.uptodate.com ↵
- Cedars, A. M. (2025). Transposition of the great arteries (TGA). Johns Hopkins Medicine. https://www.hopkinsmedicine.org/health/conditions-and-diseases/transposition-of-the-great-arteries-tga ↵
- Cedars, A. M. (2025). Transposition of the great arteries (TGA). Johns Hopkins Medicine. https://www.hopkinsmedicine.org/health/conditions-and-diseases/transposition-of-the-great-arteries-tga ↵
- Centers for Disease Control. (2024, October 4). About hypoplastic left heart syndrome. https://www.cdc.gov/heart-defects/about/hypoplastic-left-heart-syndrome.html ↵
- Centers for Disease Control. (2024, October 4). About hypoplastic left heart syndrome. https://www.cdc.gov/heart-defects/about/hypoplastic-left-heart-syndrome.html ↵
- Centers for Disease Control. (2024, October 4). About hypoplastic left heart syndrome. https://www.cdc.gov/heart-defects/about/hypoplastic-left-heart-syndrome.html ↵
- Centers for Disease Control. (2024, October 4). About hypoplastic left heart syndrome. https://www.cdc.gov/heart-defects/about/hypoplastic-left-heart-syndrome.html ↵
- Binks, A. (2024). 6.1: Congenital heart disease. LibreTexts: Medicine. https://med.libretexts.org/Courses/Virginia_Tech_Carilion_School_of_Medicine/Cardiovascular_Pathophysiology_for_Pre-Clinical_Students_(Binks)/06:_Congenital_Heart_Disease/6.01:_Congenital_heart_disease ↵
- “Coarctation of the aorta” by Kindred Grey is licensed under CC BY 4.0 ↵
- Binks, A. (2024). 6.1: Congenital heart disease. LibreTexts: Medicine. https://med.libretexts.org/Courses/Virginia_Tech_Carilion_School_of_Medicine/Cardiovascular_Pathophysiology_for_Pre-Clinical_Students_(Binks)/06:_Congenital_Heart_Disease/6.01:_Congenital_heart_disease ↵
- Betts, J. G., Desaix, P., Johnson, E., Johnson, J. E., Korol, O., Kruse, D., Poe, B., Wise, J., Womble, M. D., & Young, K. A. (2022). Anatomy and physiology, 2e. OpenStax. https://openstax.org/details/books/anatomy-and-physiology-2e ↵
- “Blausen_0648_MitralValveStenosis” by Blausen Medical Communications, Inc. is licensed under CC BY 3.0 ↵
- del Nido, P. J., & Baird, C. (2012). Congenital mitral valve stenosis: Anatomic variants and surgical reconstruction. Seminars in Thoracic and Cardiovascular Surgery: Pediatric Cardiac Surgery Annual, 15(1), 69–74. https://doi.org/10.1053/j.pcsu.2012.01.011 ↵
- Boston Children’s Hospital. (n.d.). Mitral valve stenosis. https://www.childrenshospital.org/conditions/mitral-valve-stenosis ↵
- Boston Children’s Hospital. (n.d.). Mitral valve stenosis. https://www.childrenshospital.org/conditions/mitral-valve-stenosis ↵
Structural abnormalities in the heart or blood vessels that are present at birth and affect how blood flows through the heart and out to the rest of the body.
Heart defects that involve right to left shunting of blood through the heart, thus bypassing the lungs and resulting in low oxygen blood being pumped throughout the body.
Congenital heart defects that do not significantly affect oxygenation but may lead to other circulatory problems.
A congenital heart defect in which the ductus arteriosus fails to close at birth.
An increased difference between systolic and diastolic blood pressure readings.
A congenital heart defect which occurs when there is a hole in the septum which separates the right and left atria of the heart. This defect allows blood to shunt from the left atrium to the right atrium.
A muscular wall that separates the atria or ventricles of the heart.
An opening between the right and left atria that fails to close at birth when the infant takes its first breaths and pressures within the atria begin to rise.
A blowing or whooshing sound that signifies turbulent blood flow.
A heart defect occurring when there is a hole in the septum separating the right and left ventricles that allows blood to shunt from the left to right ventricle.
Heart defects that involve right to left shunting of blood through the heart, thus bypassing the lungs and resulting in low oxygen blood being pumped throughout the body.
Elevated number of red blood cells.
A severe episode of hypoxia that typically occurs in clients with Tetralogy of Fallot, also known as a “Tet Spell.” During these episodes, there is a significant decrease in blood flow moving to the lungs due to a sudden increase in shunting of blood from the right side to left side of the heart. Deoxygenated blood is pumped out to the body, resulting in signs and symptoms of severe hypoxia.
Directing a flow of oxygen at high concentration near the client's face without making direct contact.
A combination of four different congenital heart defects in which there is a ventral septal defect, hypertrophy of the right ventricle, narrowing of the pulmonic valve, and a displaced aorta.
A congenital heart defect in which the positions of the pulmonary artery and the aorta are switched, resulting in abnormal circulation of blood.
A congenital heart defect characterized by underdevelopment of the left side of the heart, including the left ventricle, left atrium, mitral valve, and aortic valve.
Heart defects that cause a restriction, or obstruction, in blood flow from within the left side of the heart or out through the body.
A condition also called critical aortic stenosis where there is an abnormal narrowing, or constriction, of the aorta.
A narrowing of the mitral valve, located between the left atrium and left ventricle, which is present at birth.

