3.3 Transport Across the Cell Membrane
Transport Across the Cell Membrane[1]
One of the great wonders of the cell membrane is its ability to regulate the amount of substances inside the cell. These substances include the following:
- Ions, such as calcium (Ca2+), sodium (Na+), potassium (K+), and chloride (Cl–)
- Nutrients, including sugars, fatty acids, and amino acids
- Wastes that must leave the cell, such as carbon dioxide (CO2)
The membrane’s phospholipid bilayer provides the first level of control. The phospholipids are tightly packed together, and the membrane has a hydrophobic (nonpolar) interior. This allows the membrane to be selectively permeable (or semipermeable) and only allow small, nonpolar, fat-soluble materials to move across it. Some examples of these materials are other lipids, oxygen and carbon dioxide gases, and alcohol.
Water-soluble materials, such as glucose, amino acids, and electrolytes, need assistance to cross the membrane because they are repelled by the hydrophobic tails in the middle of the cell membrane.
All substances that move through the membrane do so by either passive or active transport.
- Passive transport is the movement of substances across the membrane without the use of energy. For example, when riding a bike down a hill, no energy is needed to move the bike. You can coast down the hill without pedaling the bike. This is similar to passive transport of substances across the membrane. No input of energy is required to move the substance across the membrane.
- Active transport is the movement of substances across the membrane using an energy molecule called adenosine triphosphate (ATP). For example, riding a bike uphill requires energy. You have to pedal the bike to get up the hill. This is similar to active transport of substances across the membrane. Energy is also required to move the substance across the membrane.
Passive Transport
To understand how substances move passively (without energy) across a cell membrane, it is necessary to understand concentration gradients and diffusion.
A concentration gradient is the difference in concentration (amount) of a substance from one area to another. Molecules are always in motion and will move from where they are more concentrated to where they are less concentrated until they are equally distributed. When molecules move from an area of greater concentration to lower concentration, they are said to move down their concentration gradient.
Diffusion
Diffusion (or simple diffusion) is the movement of a substance from an area of higher concentration to an area of lower concentration. For example, imagine being inside a closed bathroom. If a bottle of perfume were sprayed, the scent molecules would naturally diffuse from the spot where they left the bottle to all corners of the bathroom, and this diffusion would continue until all of the perfume molecules were evenly distributed. Another example of diffusion is when a spoonful of sugar is placed in a cup of tea. Eventually, the sugar will diffuse throughout the tea until it is evenly distributed. See Figure 3.4[2] for an illustration of diffusion of small molecules across a cell membrane, from an area of higher concentration to lower concentration, over a period of time.
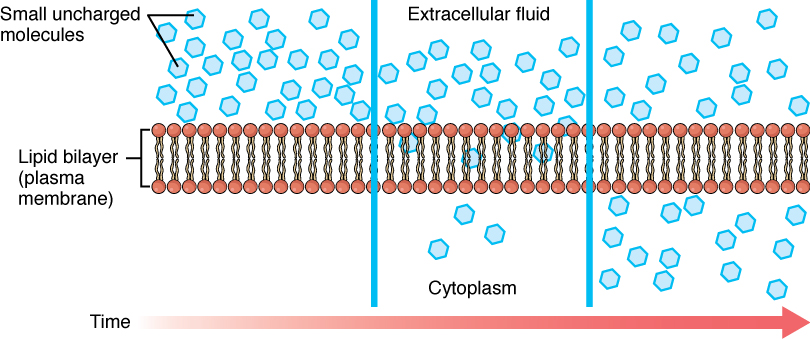
Heat increases the speed of diffusion. In both of the previous examples, if the room is warmer or the tea is hotter, diffusion occurs even faster. The human body has a body temperature around 98.6° F, which naturally helps diffusion occur within the body.
Substances that easily move across the selectively permeable cell membrane, such as oxygen (O2) and carbon dioxide (CO2), easily diffuse down their concentration gradient. Oxygen (O2) diffuses into cells because it is more concentrated outside of the cell, and carbon dioxide (CO2) diffuses out of the cell because it is more concentrated inside. These are both examples of passive diffusion because no energy is required.
Very small polar molecules, such as water, can go through the cell membrane by simple diffusion because they are so small. However, large polar or ionic molecules, which are hydrophilic (water-loving), cannot easily cross the cell membrane via simple diffusion as they are repelled by the hydrophobic fatty acid tails inside the phospholipid bilayer. These molecules need assistance to get into the cell.
Facilitated diffusion is used for substances that cannot cross the cell membrane on their own due to their size, charge, and/or polarity. Carrier proteins and channel proteins are used for facilitated diffusion to help move substances across the membrane from a high concentration to a low concentration. See Figure 3.5[3] for an illustration of facilitated diffusion.
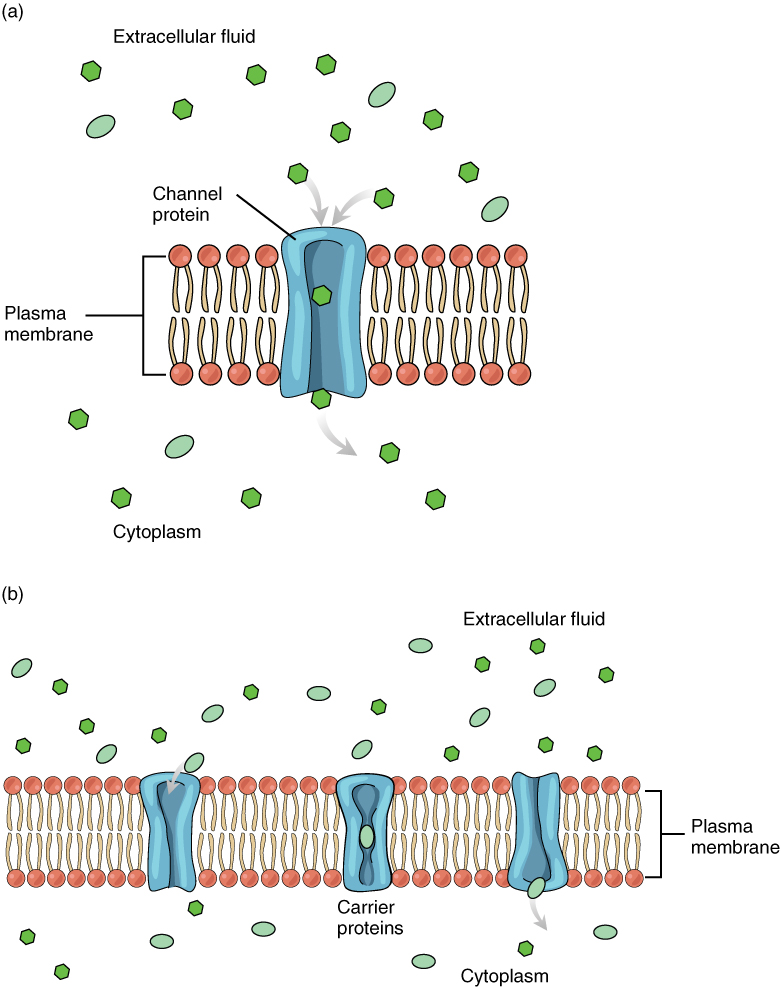
An example of a substance that uses facilitated diffusion to get into a cell via channel proteins is sodium. Even though sodium ions (Na+) are highly concentrated outside of cells, they are charged so they cannot pass directly through the cell membrane. Their diffusion is facilitated by proteins that form sodium channels (also called “pores”).
An example of facilitated diffusion via a carrier protein is movement of glucose (blood sugar) into the cell, where it is used to make ATP. Although glucose is more concentrated outside of a cell, it cannot cross the phospholipid bilayer by simple diffusion because it is both large and polar. A specialized carrier protein called a glucose transporter helps facilitate its diffusion into the cell.
There are many other substances that use facilitated diffusion to move into a cell (such as amino acids) or out of a cell (such as wastes). Because facilitated diffusion is a passive process, it does not require energy.
View a supplementary YouTube video[4] on cell transport: Cell Transport.
Osmosis
Osmosis is the diffusion of water through a selectively permeable membrane from an area of high concentration of water molecules to an area of low concentration of water molecules. In other words, water moves from a dilute or watery environment towards a concentrated (“saltier”) environment, commonly referred to as “water follows salt.” The less concentrated or dilute environment has more water and less solute present in its solution whereas the concentrated solution has more solute dissolved in it. Water easily moves across cell membranes through protein channels or by simply slipping between the lipid tails of the membrane itself.
Isotonic Solutions
Because some cells cannot regulate the movement of water on their own, these cells must be in an environment where the concentration of solutes outside of the cells (in the extracellular fluid) is equal to the concentration of solutes inside the cells (in the cytosol or intracellular fluid). Solutions that have the same concentration of solutes as the concentration inside the cell are said to be isotonic (i.e., same or equal tension). When the cell is in an isotonic environment, water will enter and leave the cell at the same rate and there will be no overall net gain or loss of water. The cell maintains its normal shape and function.
An important part of homeostasis is to create an environment in which all of the body’s cells are in an isotonic solution. Several organs in the body, particularly the kidneys, work to maintain this homeostasis.
Hypertonic and Hypotonic Solutions
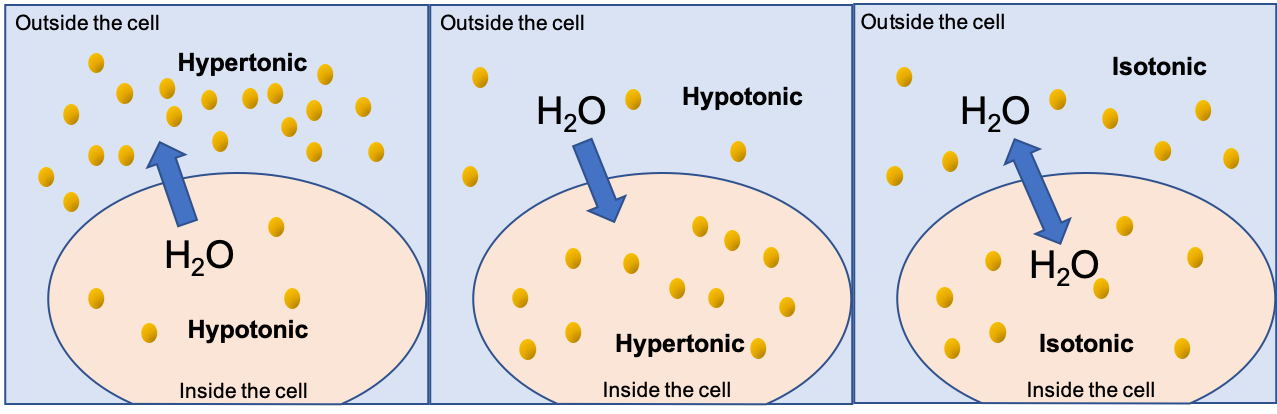
When the concentration of solutes outside of the cell is different from the concentration of the solutes inside the cell, water will move from one side of the cell membrane to the other to try and equalize the two concentrations during osmosis.
A solution that has a higher concentration of solutes than another solution is said to be hypertonic, and water molecules tend to diffuse into a hypertonic solution. Cells in a hypertonic solution will shrivel (crenate) if too much water leaves the cell via osmosis. In contrast, a solution that has a lower concentration of solutes than another solution is said to be hypotonic, and water molecules tend to diffuse out of a hypotonic solution. Cells in a hypotonic solution will take on too much water and swell, with the risk of eventually bursting (lysing). See Figure 3.6[5] for an illustration of the movement of water into and out of cells in isotonic, hypertonic, and hypotonic solutions.
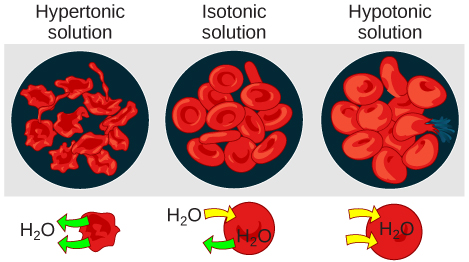
Clinical Connection
Intravenous fluids are commonly administered to hospitalized patients. It is important for nurses and health care providers to understand the effects of osmosis on the patient’s cells as a result of the concentration of the administered fluids. For example if a hypertonic intravenous solution is administered into a patient’s bloodstream, water will move by osmosis out of their cells and into the bloodstream, causing the cells to shrivel. Conversely, if a hypotonic intravenous solution is administered, water will move by osmosis from the bloodstream into the cells, causing them to swell. These actions can be easily remembered with the phrase “water follows salt,” meaning water will move from an area of lower concentration of solutes to an area of higher concentration of solutes. See Figure 3.7[6] for an illustration of the effects of the intravenous administration of hypertonic and hypotonic fluid on red blood cells.
Active Transport
Recall that diffusion causes movement of substances from an area of higher concentration to an area of lower concentration, which is called a concentration gradient. Active transport is used to move a substance against its concentration gradient across the cell membrane, often with the help of protein carriers. Active transport requires the use of energy in the form of ATP.
Pumps
One of the most common types of active transport uses proteins that work as pumps. The word “pump” probably brings up thoughts of using energy to pump up the tire of a bicycle or a basketball. Similarly, energy from ATP is required for cell membrane proteins to transport substances (i.e., molecules or ions) across the membrane against their concentration gradients (i.e., from an area of low concentration to an area of high concentration).
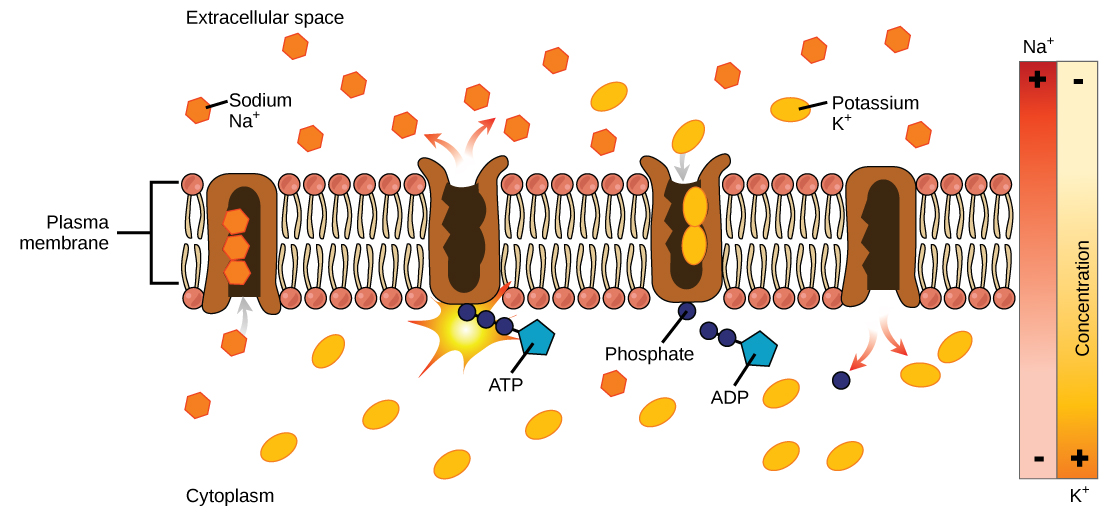
An example of active transport is the sodium-potassium pump, which moves sodium out of a cell while moving potassium into the cell. The sodium-potassium pump is an important ion pump found in the membranes of many types of cells. See Figure 3.8[10] for an image of the sodium-potassium pump.
Other Types of Active Transport
Endocytosis
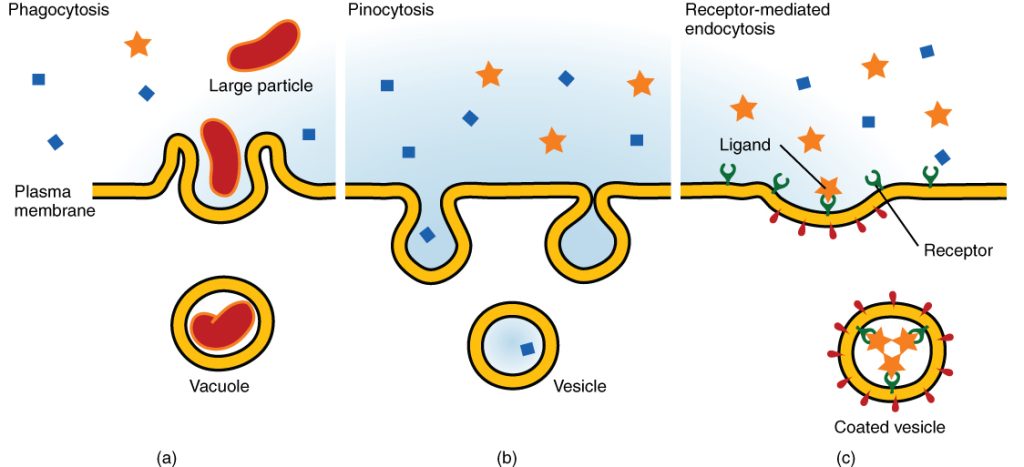
Endocytosis (which means bringing “into the cell”) is another type of active transport where cells take in materials from outside the cell. During endocytosis, the cell ingests material by enveloping it in a portion of its cell membrane and then pinching off that portion. Once pinched off, that portion of the membrane and its contents become an independent, intracellular vesicle. A vesicle is a membrane sac (i.e., a round and hollow organelle bounded by part of the cell membrane). Endocytosis often brings materials into the cell that must be broken down or digested.
There are three different types of endocytosis, which include phagocytosis, pinocytosis, and receptor-mediated endocytosis. See Figure 3.9[11] for an illustration of these types of endocytosis. Phagocytosis and pinocytosis bring in large portions of extracellular material, and they usually are not selective in the substances they bring into the cell. Phagocytosis (which means “cell eating”) refers to endocytosis of large particles. For example, white blood cells patrol the body for unwanted foreign objects, such as invading bacteria, phagocytize them, and then digest them. Pinocytosis (which means “cell drinking”) is the endocytosis of fluids. Receptor-mediated endocytosis regulates the endocytosis of specific substances by using a part of the cell membrane that has receptors for a certain substance. The substance being ingested has to attach to the cell’s receptor first before it is engulfed by the cell. For example, iron is brought into red blood cells using receptor-mediated endocytosis.
Exocytosis
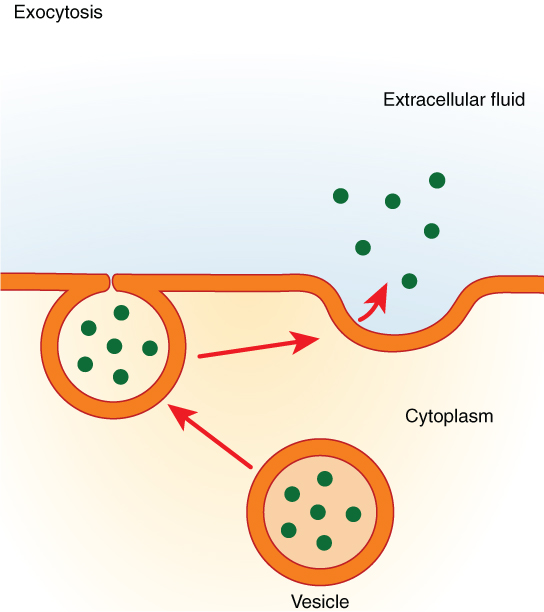
Exocytosis (which means “exiting or leaving the cell”) is the opposite of endocytosis. Many cells make things that must be removed from the cell, like a factory makes a product for export. These substances are typically packaged into vesicles while inside of the cell. The vesicle then merges with the cell membrane and releases its contents outside of the cell. For example, cells in the stomach and pancreas secrete digestive enzymes through exocytosis to help break down the food eaten. See Figure 3.10[12] for an illustration of exocytosis.
- Betts, J. G., Desaix, P., Johnson, E., Johnson, J. E., Korol, O., Kruse, D., Poe, B., Wise, J., Womble, M. D., & Young, K. A. (2022). Anatomy and physiology, 2e. OpenStax. https://openstax.org/books/anatomy-and-physiology-2e/pages/1-introduction ↵
- “0305_Simple_Diffusion_Across_Plasma_Membrane” by OpenStax is licensed under CC BY 4.0 ↵
- “0306_Facilitated_Diffusion” by OpenStax is licensed under CC BY 4.0 ↵
- Amoeba Sisters. (2016, June 24). Cell transport [Video]. YouTube. All rights reserved. https://www.youtube.com/watch?v=Ptmlvtei8hw ↵
- “Cellular_Tonicity” by Connectivid-D is licensed under CC BY-SA 4.0 ↵
- “Figure_05_02_07” by CNX OpenStax is licensed under CC BY 4.0 ↵
- BOGObiology. (2018, October 28). Hypertonic, hypotonic, and isotonic solutions! [Video]. YouTube. All rights reserved. https://www.youtube.com/watch?v=rMa9MzP19zI ↵
- MooMooMath and Science. (2020, September 25). Types of solutions-isotonic-hypertonic-hypotonic-animation [Video]. YouTube. All rights reserves. https://www.youtube.com/watch?v=H2dBFiBOOGY ↵
- Ameoba Sisters. (2018, June 27). Osmosis and water potential (updated) [Video]. YouTube. All rights reserved. https://www.youtube.com/watch?v=L-osEc07vMs ↵
- “Figure_05_03_03” by CNX OpenStax is licensed under CC BY 4.0 ↵
- “0309_Three_Forms_of_Endocytosis” by OpenStax is licensed under CC BY 4.0 ↵
- “0310_Exocytosis” by OpenStax is licensed under CC BY 4.0 ↵
Charged particles (positively or negatively) that are essential for cellular processes, such as nerve signaling and muscle contraction (e.g., Na⁺, K⁺, Ca²⁺, Cl⁻).
Substances needed for cell survival and function, including carbohydrates, proteins, fats, vitamins, and minerals.
Unwanted byproducts of cellular metabolism that must be removed to maintain homeostasis, such as carbon dioxide (CO₂) and urea.
A property of the cell membrane that allows certain molecules to pass through while restricting others, maintaining internal balance, also known as semipermeable,
A property of the cell membrane that allows certain molecules to pass through while restricting others, maintaining internal balance, also known as selectively permeable.
Movement of molecules across the cell membrane without energy input, following their concentration gradient (high to low concentration).
Movement of molecules across the membrane requiring energy (ATP) to move substances against their concentration gradient (low to high concentration).
A difference in the concentration of a substance across a space or membrane, driving diffusion and osmosis.
The passive movement of molecules from an area of higher concentration to lower concentration until equilibrium is reached.
A type of diffusion where small, nonpolar molecules (e.g., oxygen, carbon dioxide) move directly through the phospholipid bilayer without assistance.
A type of passive transport where molecules (e.g., glucose, ions) move across the membrane with the help of carrier or channel proteins.
A simple sugar.
A membrane protein that facilitates the movement of glucose into the cell through facilitated diffusion or active transport.
The diffusion of water across a selectively permeable membrane from an area of low solute concentration to high solute concentration.
A solution where the concentration of solutes is equal inside and outside the cell, resulting in no net water movement.
A solution with a higher solute concentration outside the cell, causing water to move out of the cell, leading to cell shrinkage (crenation in animal cells).
A solution with a lower solute concentration outside the cell, causing water to move into the cell, which may lead to cell swelling or bursting (lysis in animal cells).
The rupturing of a cell due to excessive water intake in a hypotonic environment.
A membrane protein that uses ATP to actively transport 3 Na⁺ ions out and 2 K⁺ ions into the cell, essential for nerve impulses and muscle contractions.
A process where the cell engulfs substances by forming a vesicle, allowing the intake of large molecules.
A small, membrane-bound sac that transports substances within or outside the cell, involved in endocytosis and exocytosis.
A type of endocytosis where the cell engulfs large particles or microorganisms ("cell eating"), commonly performed by immune cells like macrophages.
A type of endocytosis where the cell engulfs liquids and dissolved nutrients ("cell drinking").
A specialized form of endocytosis where specific molecules bind to cell-surface receptors, triggering vesicle formation to bring them inside.
A process where vesicles fuse with the plasma membrane to release substances outside the cell, used for removing waste or secreting proteins.

