2.3 Chemical Bonds
Chemical Bonds[1],[2]
As discussed previously, most elements don’t have enough electrons to fill their outermost shells, but an atom is most stable when all the electron positions in the outermost shell are filled. The way that atoms fill their outermost electron shells is by the formation of chemical bonds. A bond is a weak or strong electrical attraction that holds atoms in the same area. The new grouping is more stable or less likely to react again. A stable grouping of two or more atoms held together by chemical bonds is called a molecule. The bonded atoms may be of the same element, as in H2, which is called molecular hydrogen or hydrogen gas. When a molecule is made up of two or more atoms of different elements, it is called a chemical compound. A unit of water, or H2O, is a compound, as is a single molecule of the gas methane, or CH4.
Three types of chemical bonds are important in human physiology because they hold together substances that are used by the body to maintain homeostasis. These are ionic bonds, covalent bonds, and hydrogen bonds.
Ions and Ionic Bonds
Ions are atoms or molecules with an electrical charge. When an atom does not contain equal numbers of protons and electrons, it is called an ion. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in its valence shell.
For example, potassium (K) is an important element in all body cells. Its atomic number is 19, so there are 19 protons and 19 electrons: 2 in the first shell, 8 in the second shell, 8 in the third shell, and just one electron in its valence shell. Because its valence shell is not full, potassium is highly likely to participate in chemical reactions to donate that one electron. (It is easier for potassium to donate one electron than to gain seven electrons.) The loss will result in potassium having one more proton than electron, so it will no longer be neutral, but instead have a slightly positive charge. A potassium ion with a positive charge is a cation, written K+, indicating that it has lost a single electron.
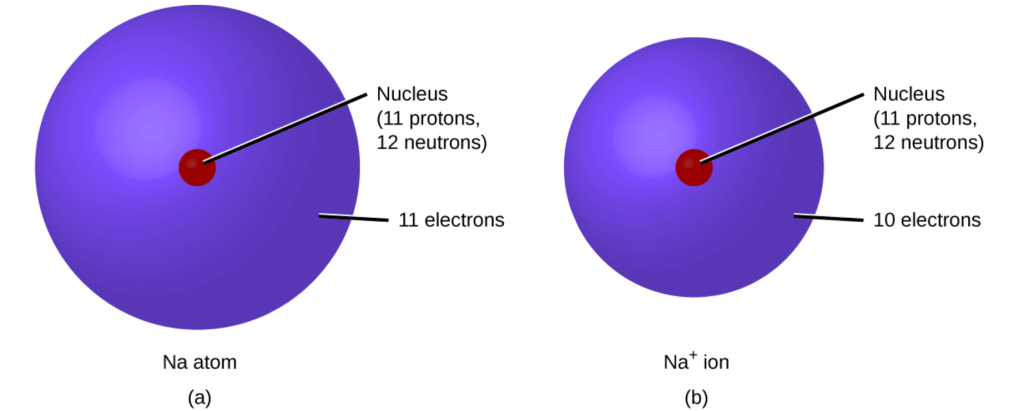
In another example, sodium only has one electron in its outermost shell. It takes less energy for sodium to donate that one electron than it does to accept seven more electrons to fill the outer shell. If sodium loses an electron, it now has 11 protons and only 10 electrons, leaving it with an overall charge of +1. It is now a cation and written as Na+. See Figure 2.7[3] for an illustration of a sodium atom compared to a sodium ion.
As previously mentioned, atoms can also gain an electron or electrons and become an anion (negatively charged). An example of this is fluorine (F), an important element in bones and teeth. Its atomic number is 9, so it has 9 protons and 9 electrons: 2 electrons in the first shell and seven electrons in its valence shell. Therefore, fluorine is likely to bond with other atoms where it gains one electron to fill its valence shell (it is easier for fluorine to gain one electron than to donate seven electrons). When it does, its electrons will outnumber its protons by one, and it will have an overall negative charge. The ionized form of fluorine is called fluoride and is written as F–.
Another example of gaining an electron is the chlorine atom. It has seven electrons in its outer shell. Again, it is more energy efficient for chlorine to gain one electron than to lose seven. Therefore, it tends to gain an electron to create an ion with 17 protons and 18 electrons, giving it a net negative (–1) charge. It is now called a chloride ion and written as Cl–. This movement of electrons from one element to another is referred to as electron transfer.
Atoms that have more than one electron to donate or accept will end up with stronger positive or negative charges. A cation that has donated two electrons has a net charge of +2. Using magnesium (Mg) as an example, this can be written Mg++ or Mg2+. An anion that has accepted two electrons has a net charge of –2. The ionic form of selenium (Se), for example, is typically written Se2–.
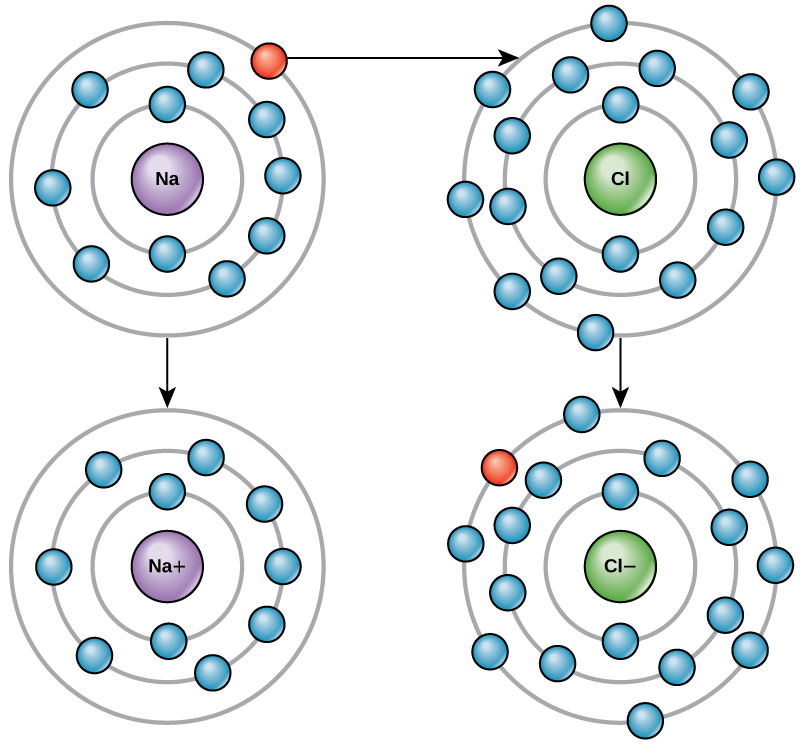
Figure 2.8[4] illustrates the transfer of electrons. A sodium atom (Na) only has one electron in its outermost shell. A chlorine atom (Cl) has seven electrons in its outermost shell. A sodium atom will donate its one electron to empty its shell, and a chlorine atom will accept that electron to fill its shell, becoming chloride. Both ions now satisfy the octet rule and have complete outermost shells. Because the number of electrons is no longer equal to the number of protons, each is now an ion and has a +1 (sodium) or –1 (chloride) charge. These two ions are then strongly attracted to each other to form the ionic compound, NaCl, better known as table salt. The attraction of many sodium and chloride ions results in the formation of a large grouping of ions called crystals.
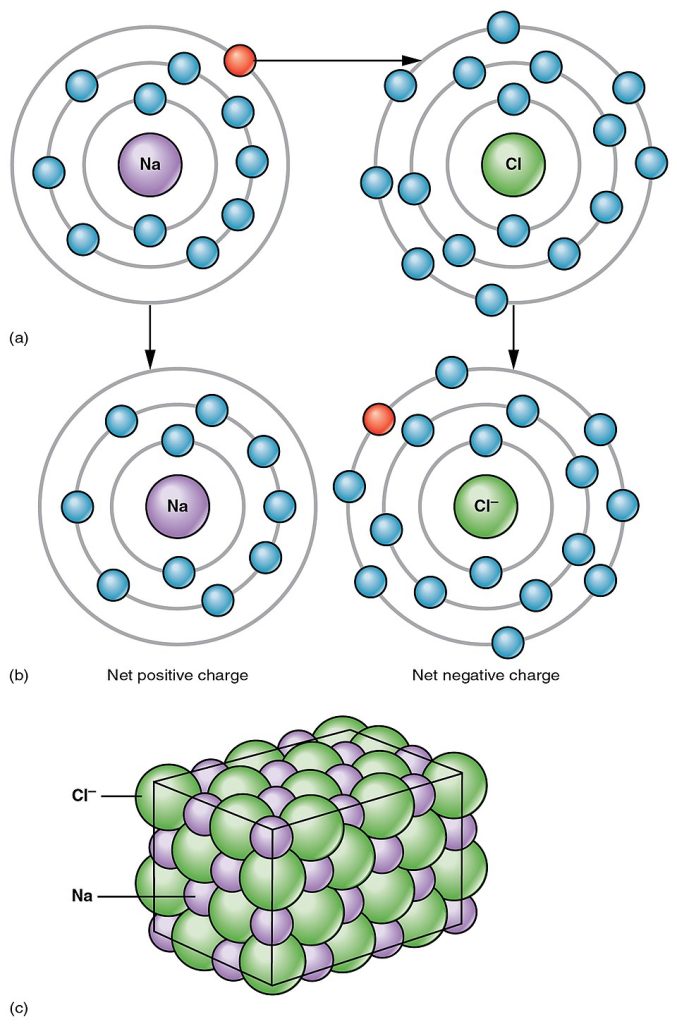
Compounds composed of ions are called ionic compounds (or salts), and their ions are held together by ionic bonds. An ionic bond is a chemical bond formed between a cation and anion. The opposite electrical charges create a moderately strong attraction and keep the atoms close to each other. The table salt you sprinkle on your food owes its existence to ionic bonding. As shown in Figure 2.9,[5] sodium commonly donates an electron to chlorine, becoming the cation Na+. When chlorine accepts the electron, it becomes the chloride anion, Cl–. With their opposing charges, these two ions strongly attract each other.
Most ionic compounds dissolve easily in water. Water is an essential component of life because it is able to break the ionic bonds in salts to free the ions. In fact, in body fluids, most individual atoms exist as ions. These dissolved ions produce electrical charges in the heart and brain, which are observed as waves on an electrocardiogram (EKG or ECG) or an electroencephalogram (EEG).
The electrical activity that comes from the interactions of the charged ions is why they are also called electrolytes. Electrolytes, such as sodium (Na+) and potassium (K+), are important as they are used to send nerve signals and also used in muscle contraction, including the beating of the heart. When electrolyte levels are out of homeostatic balance in the body, it can be life-threatening.
Covalent Bonds
Another type of strong chemical bond between two or more atoms is a covalent bond. Covalent bonds form when electrons are shared between two elements. Just like neighbors whose kids hang out at one home and then at the other, the atoms do not lose or gain electrons permanently. Instead, the electrons move back and forth between the elements. They are the strongest and most common form of chemical bond in living organisms. Covalent bonds form between the elements that make up the biological molecules in our cells. Unlike ionic bonds, covalent bonds do not dissociate or break apart in water.
The hydrogen and oxygen atoms that combine to form water molecules are held together with covalent bonds. The electron from the hydrogen atom divides its time between the incomplete outer shell of the hydrogen atom and the incomplete outer shell of the oxygen atom. To completely fill the outer shell of an oxygen atom, two electrons from two hydrogen atoms are needed, hence the subscript “2” in H2O. The electrons are shared between the atoms, dividing their time between them to “fill” the outer shells of each.
Polar and Nonpolar Covalent Bonds
There are two types of covalent bonds: nonpolar and polar. Nonpolar covalent bonds form between two atoms of the same element or between different elements that share the electrons equally. For example, an oxygen atom can bond with another oxygen atom to fill both of their outer electron shells. This association is nonpolar because the electrons will be equally distributed or shared between each oxygen atom. If two atoms share one pair (or two individual) of electrons, then it forms a single bond. In the case of O2, two pairs of electrons are shared between them, which forms a double bond. A triple bond forms when three pairs of electrons are shared between atoms. A triple bond is found in N2 gas. Another example of a nonpolar covalent bond is found in the methane (CH4) molecule. These elements all share the electrons equally, creating four nonpolar covalent bonds.
See Figure 2.10[6] for illustrations of several common types of covalent bonds. Two covalently bonded atoms usually share just one or two electron pairs. In covalent bonds, electrons in the two atoms’ overlapping orbits are shared to fill the valence shells of both atoms, stabilizing both.
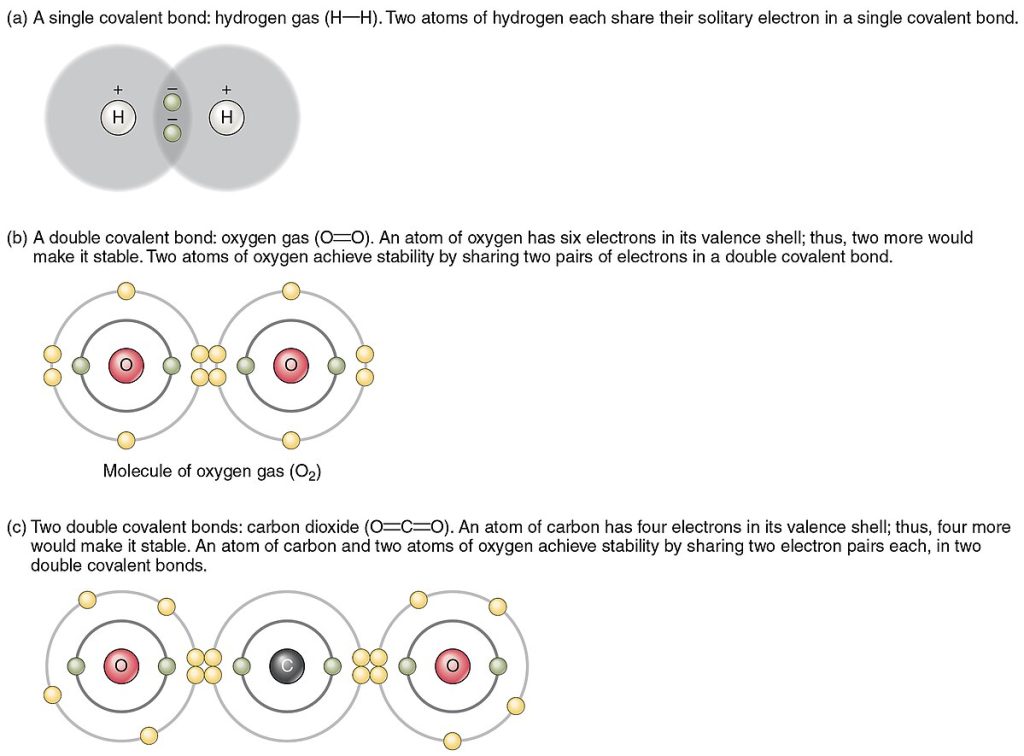
In a single covalent bond, a single electron pair is shared between two atoms, while in a double covalent bond, two pairs of electrons are shared between two atoms. There even are triple covalent bonds, where three electron pairs are shared between two atoms.
The covalent bonds shown in Figure 2.10 are balanced. The sharing of the negative electrons is relatively equal, as is the electrical pull of the positive protons in the nucleus of the atoms involved. This is why covalently bonded molecules that are electrically balanced in this way are described as nonpolar; that is, no part is either more positive or more negative than any other.
See Figure 2.11[7] for another illustration of single and double bonds.
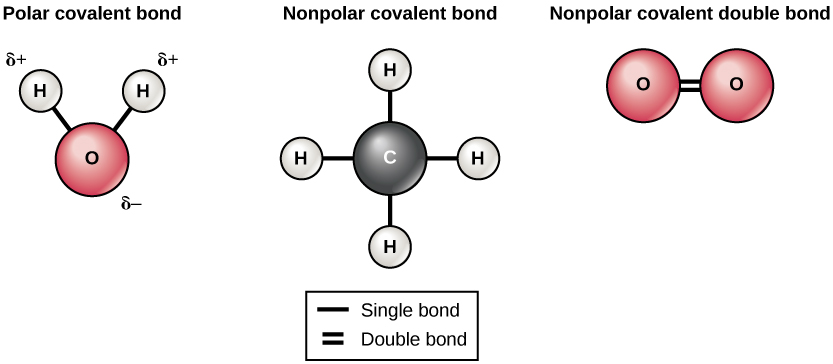
Unlike nonpolar bonds, polar covalent bonds are formed between two or more different elements that do not share electrons equally. This unequal sharing occurs when one atom “hogs” the electrons in the molecule. This happens when an element is more electronegative than another one and gives it a stronger attractive force pulling the shared electron closer to itself. The water molecule is an example of a molecule with a polar bond with a slight positive charge on the hydrogen atoms and a slight negative charge on the oxygen. This happens because oxygen is more electronegative than hydrogen and will pull the shared electrons closer to itself (and away from hydrogen).
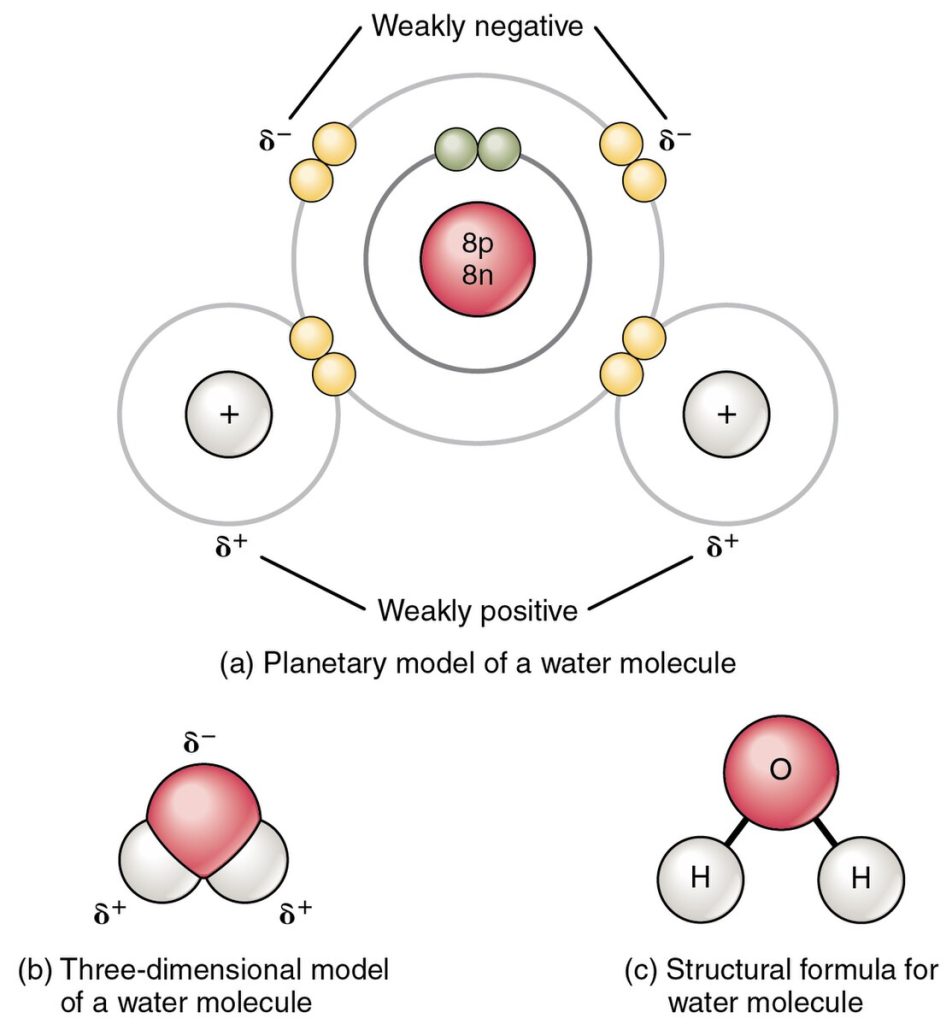
In a polar covalent bond, the electrons shared by the atoms spend more time closer to one nucleus than to the other nucleus. Because of the unequal distribution of electrons between the different nuclei, a slightly positive (δ+) or slightly negative (δ–) charge develops. The covalent bonds between hydrogen and oxygen atoms in water are polar covalent bonds. The shared electrons spend more time near the oxygen nucleus, giving it a small negative charge, than they spend near the hydrogen nuclei, giving these molecules a small positive charge. See Figure 2.12[8] for an illustration of polar covalent bonds in a water molecule.
Hydrogen Bonds
Ionic and covalent bonds are strong bonds that require considerable energy to break. However, not all bonds between elements are ionic or covalent bonds. Weaker bonds can also form. These are attractions that occur between positive and negative charges in polar covalent molecules that do not require much energy to break. One common example is a hydrogen bond. These bonds give rise to the unique properties of water and the unique structures of DNA and proteins.
The most common example of hydrogen bonding occurs between molecules of water. Hydrogen bonding in water molecules occurs because the weakly positive hydrogen atoms are attracted to the weakly negative oxygen atoms in different water molecules. Hydrogen bonding between water molecules is what causes water molecules to be cohesive or “stick” together. Hydrogen bonds give water the unique properties that sustain life. If it were not for hydrogen bonding, water would be a gas rather than a liquid at room temperature. See Figure 2.13[9] for an illustration of hydrogen bonds between water molecules. Notice that the bonds occur between the weakly positive charge on the hydrogen atoms and the weakly negative charge on the oxygen atoms. Hydrogen bonds are weak bonds and are indicated with a dotted (rather than a solid) line.
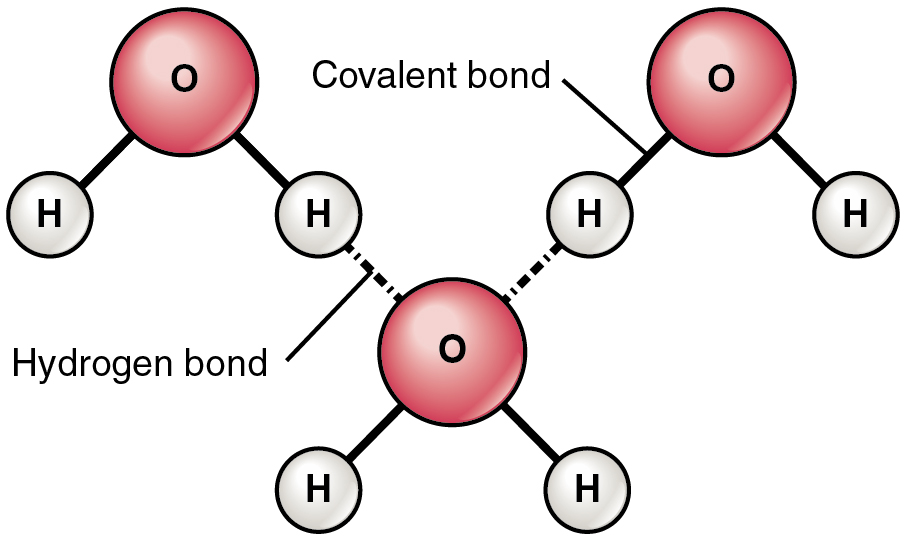
Hydrogen bonds can form between different molecules, and they do not always have to include a water molecule. Hydrogen atoms in polar bonds within any molecule can form bonds with other nearby molecules. For example, hydrogen bonds hold together two long strands of DNA to give the DNA molecule its characteristic double-stranded helix structure. Hydrogen bonds are also responsible for some of the three-dimensional structure of proteins.
- Betts, J. G., Desaix, P., Johnson, E., Johnson, J. E., Korol, O., Kruse, D., Poe, B., Wise, J., Womble, M. D., & Young, K. A. (2022). Anatomy and physiology, 2e. OpenStax. https://openstax.org/books/anatomy-and-physiology-2e/pages/1-introduction ↵
- Fowler, S., Roush, R., & Wise, J. (2013). Concepts of biology. OpenStax. https://openstax.org/books/concepts-biology/pages/1-introduction ↵
- “5f15c723c3300cd09b4db14bef2d78cd6e72a8b0” by Rice University is licensed under CC BY-NC 4.0. Access for free at https://openstax.org/books/chemistry-atoms-first-2e/pages/3-7-ionic-and-molecular-compounds#CNX_Chem_02_06_NaCation ↵
- “17ddc116f656484228b3ae5263a4fa27052b0bb9” by OpenStax is licensed under CC BY 4.0. Access for free at https://openstax.org/books/concepts-biology/pages/2-1-the-building-blocks-of-molecules ↵
- “207_Ionic_Bonding-01” by OpenStax College is licensed under CC BY 3.0 ↵
- “208_Covalent_Bonding-01” by OpenStax College is licensed under CC BY 3.0 ↵
- “7762f7ba5e25fc2574d74910924a76231f879c8a” by Rice University is licensed under CC BY 4.0. Access for free at https://openstax.org/books/concepts-biology/pages/2-1-the-building-blocks-of-molecules ↵
- “209_Polar_Covalent_Bonds_in_a_Water_Molecule” by OpenStax College is licensed under CC BY 3.0 ↵
- “210_Hydrogen_Bonds_Between_Water_Molecules-01” by OpenStax College is licensed under CC BY 3.0 ↵
A weak or strong electrical attraction that holds atoms in the same area.
When two or more atoms combine chemically.
A substance composed of two or more elements joined by chemical bonds.
An atom that does not contain equal numbers of protons and electrons.
A positive ion.
A negative ion.
The movement of electrons from one element to another.
Compounds composed of ions, also known as salts.
A chemical bond formed between a cation and anion.
Type of bond that forms when electrons are shared between two elements.
Type of bonds which form between two atoms of the same element or between different elements that share the electrons equally.
Type of bond in which two atoms share one pair (or two individual) electrons.
Type of bond in which two pairs of electrons are shared.
Type of bond in which three pairs of electrons are shared between atoms.
Type of bond formed between two or more different elements which do not share electrons equally.
A weak to moderate attractive force that occurs between a hydrogen atom, which is covalently bonded to a more electronegative atom (like oxygen, nitrogen, or fluorine), and another electronegative atom with a lone pair of electrons

