2.2 Elements and Compounds
Elements and Compounds[1]
All matter (anything that takes up space and has a mass) is made up of one or more substances called elements. An element is a pure substance that cannot be created or broken down by ordinary chemical means or transformed chemically into other substances.
Each element is made of atoms, the smallest amount of an element. A total of 118 elements have been defined; however, only 92 occur naturally, and fewer than 30 are found in living cells. The remaining 26 elements are unstable and, therefore, do not exist for very long or are theoretical and have yet to be detected.
The periodic table is a chart of all of the known elements. Each element has a one to two letter chemical symbol (such as H, N, O, C, and Cl) and possesses unique properties. For example, sodium’s symbol is Na. These unique properties allow elements to combine and to bond with each other in specific ways.
While your body can make many of the chemicals needed for life from elements, it cannot make elements. All the elements in your body are derived from the foods you eat and the air you breathe.
An example of an element that you must take in is calcium (Ca). Calcium is used by the body for many things, including strengthening bones. The calcium in cheese is the same as the calcium that forms your bones. Some other examples of important elements are oxygen, sodium, and iron.
There are many elements found in the human body. See Figure 2.1[2] for a complete list of elements in the human body. The four most abundant elements in the human body are as follows:
- Oxygen (O)
- Carbon (C)
- Hydrogen (H)
- Nitrogen (N)
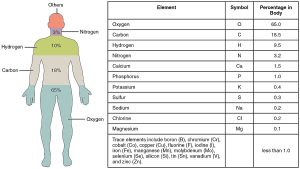
In nature, elements rarely occur alone. They usually combine to form compounds. A compound is a substance composed of two or more elements joined by chemical bonds. For example, glucose (blood sugar) is an important body fuel. It is made up of the same three elements: carbon, hydrogen, and oxygen.
Building Blocks of Molecules
Atoms and Subatomic Particles
An atom is the smallest amount of an element. For example, one atom of hydrogen is the smallest amount of hydrogen that can exist. Atoms are extremely small. In fact, the period at the end of this sentence is millions of atoms wide. At the most basic level, all organisms contain atoms that combine together to form molecules. In multicellular organisms, such as humans, molecules can interact to form cells that combine to form tissues, which make up organs and organ systems. These combinations continue until entire organisms are formed.
Atomic Structure
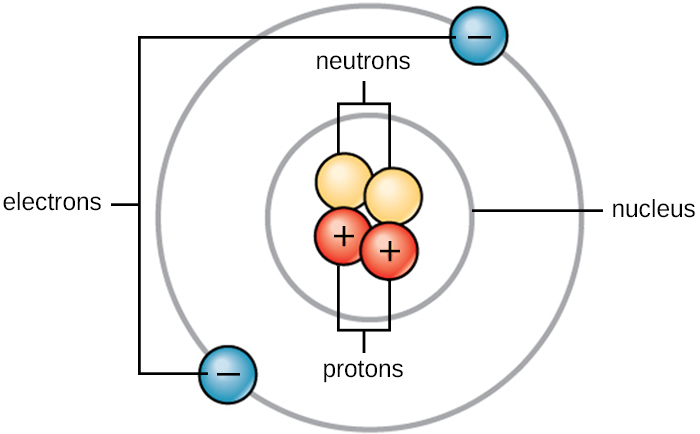
Atoms are made up of even smaller particles called subatomic particles. These include protons, neutrons, and electrons.
- Protons (p+): Protons are positively charged particles found in the nucleus or center of the atom.
- .The number of protons determines the atomic number
- For example, oxygen has 8 protons, so oxygen’s atomic number on the periodic table is 8.
- .The number of protons determines the atomic number
- Neutrons (n): Neutrons do not have a charge, so they are neutral. They have a mass of 1. They are also found in the center or nucleus of the atom.
- Electrons (e-): Electrons are very small, negatively charged particles found outside of the atom’s nucleus. They “spin” around the nucleus close to the speed of light in electron shells or clouds. It has almost no mass and has a charge of –1.
- The number of electrons is the same as the number of protons. This keeps the overall atom’s charge neutral.
- For example, oxygen has 8 positively charged protons, so it also has 8 negatively charged electrons.
- Positively charged protons attract negatively charged electrons, giving the atom structural stability and keeping the electrons close.
- The number of electrons is the same as the number of protons. This keeps the overall atom’s charge neutral.
See Figure 2.2[3] for an illustration of an atom with its subatomic particles.
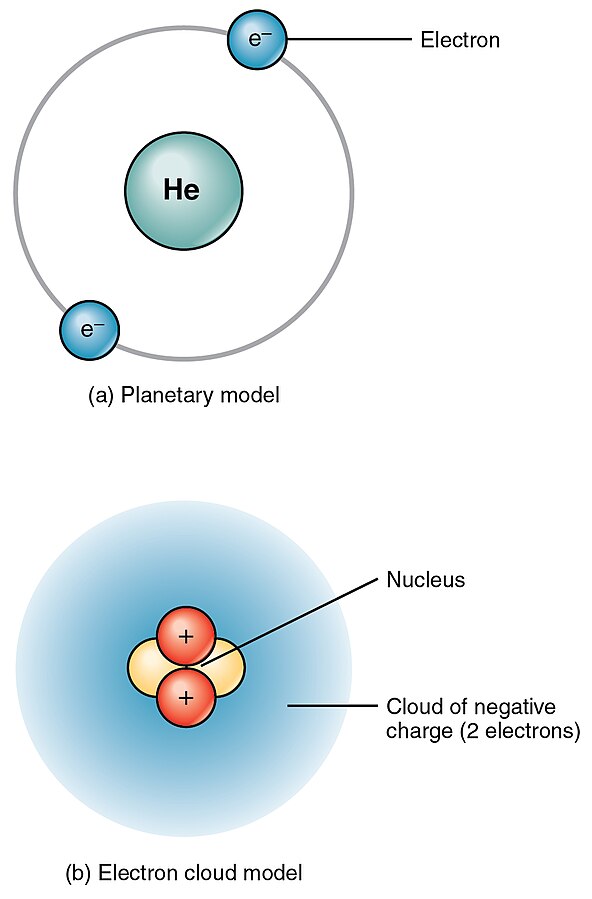
Atomic structure can be modeled in different ways. Figure 2.3[4] shows two different models of the structure of a helium (He) atom. In the first model, helium’s two electrons are shown circling the nucleus in a ring. This model is helpful for visualizing atomic structure. However, in reality, electrons do not travel in fixed rings. They spin around the nucleus in what is sometimes referred to as an electron cloud.
Atomic Number and Mass Number
Protons, neutrons and electrons are the same whether they are found in an atom of carbon (C), sodium (Na), iron (Fe), or any other element. It is the number of protons each element contains that makes it different from other elements. Therefore, the atomic number, which is the number of protons in the nucleus of the atom, identifies the element. An atom usually has the same number of electrons as protons, so the atomic number is also the number of electrons.
- Example: Carbon has the atomic number 6, telling us carbon atoms contain six protons. No other element has exactly six protons in its atoms. All atoms of carbon, whether found in your liver or in a lump of coal, contain six protons.
An element’s mass number is the total number of protons and neutrons in its nucleus. (Electrons have so little mass that they do not really contribute to the mass of an atom). Many elements contain the same number of neutrons as protons, but not all. Because protons and neutrons each have a mass of 1, the mass of an atom is equal to the number of protons and neutrons of that atom. The number of electrons does not factor into the overall mass, because their mass is so small.
- Examples:
- The most common form of carbon has six neutrons and six protons, for a mass of 12. Carbon is a relatively light element.
- Uranium (U) has a mass number of 238 and is referred to as a heavy metal. Its atomic number is 92 (it has 92 protons), but it contains 146 neutrons. Uranium has the most mass of all the naturally occurring elements.
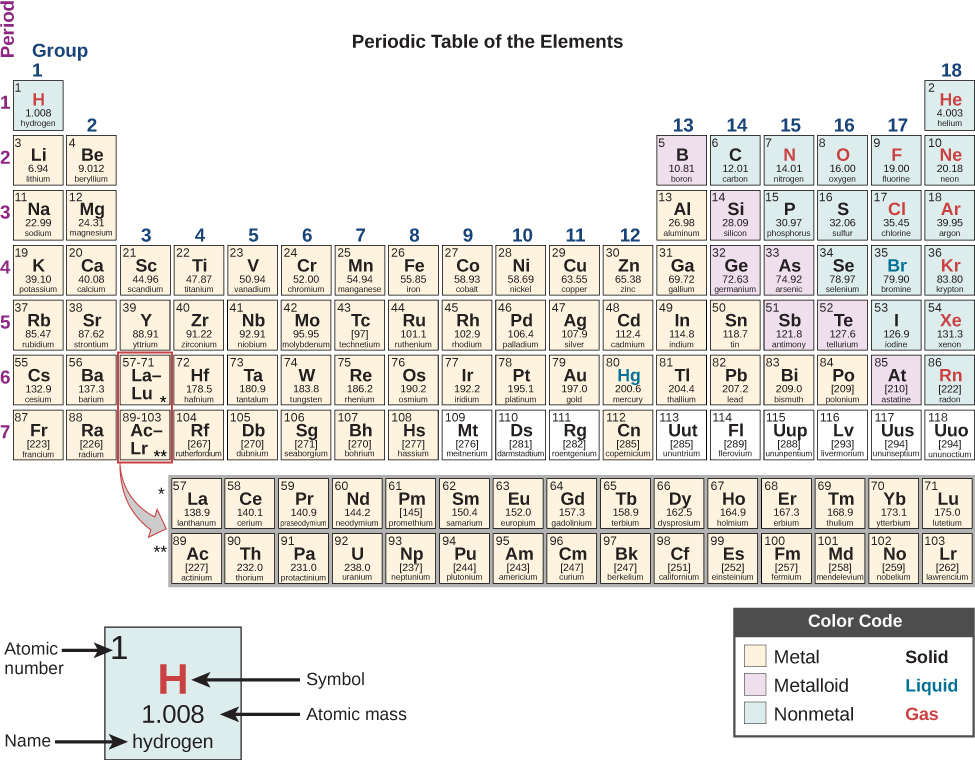
The periodic table, as shown in Figure 2.4,[5] is a chart organizing the 92 elements found in nature, as well as several elements discovered experimentally. The elements are arranged in order of their atomic number. For each element, the periodic table identifies the chemical symbol, the atomic number, and the mass number, while organizing elements according to their reactivity with other elements. The periodic table also provides key information about the properties of elements often indicated by color-coding.[6]
Isotopes
Although each element has a unique number of protons, it can exist as different isotopes. An isotope is one of the different forms of an element, distinguished from one another by different numbers of neutrons. Some elements, such as carbon, potassium, and uranium, have naturally occurring isotopes.
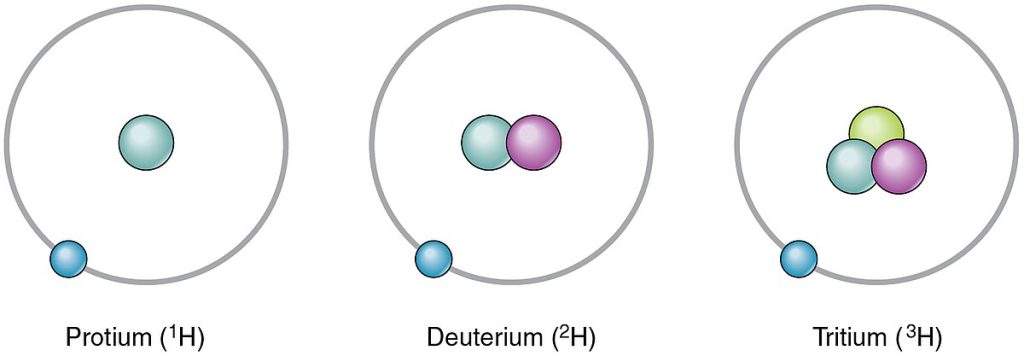
The most common isotope of carbon is 12C, commonly called carbon-12. 12C has six protons and six neutrons, for a mass number of twelve. All the isotopes of carbon have the same number of protons (six), but the number of neutrons changes. Therefore, 13C has seven neutrons, and 14C has eight neutrons. The different isotopes of an element can also be indicated with the mass number hyphenated (for example, C-12 instead of 12C). Hydrogen has three common isotopes, shown in Figure 2.5.[7] Protium, designated 1H, has one proton and no neutrons. It is by far the most common isotope of hydrogen in nature. Deuterium, designated 2H, has one proton and one neutron. Tritium, designated 3H, has one proton and two neutrons.
An isotope that contains more than the usual number of neutrons is referred to as a heavy isotope. An example is 14C. Heavy isotopes tend to be unstable, and unstable isotopes are radioactive.
A radioactive isotope (also called radioisotopes) has a nucleus that readily decays, giving off subatomic particles and electromagnetic energy. Radioactive isotopes are medically useful. They can be given to patients before medical imaging tests such as positron emission tomography or PET scans, used to destroy an overactive thyroid gland, can be used to sterilize medical equipment, and given to cancer patients as radiation therapy treatments. However, excessive exposure to radioactive isotopes can damage human cells and even cause cancer and birth defects in healthy individuals.[8]
Electrons Shells
In the human body, atoms do not exist alone. They are constantly reacting with other atoms to make or break down more complex substances. As mentioned earlier, electrons tend to stay within a certain region of space called electron clouds, sometimes referred to as an electron shell. An electron shell is a layer of electrons that circles the nucleus at a distinct energy level.
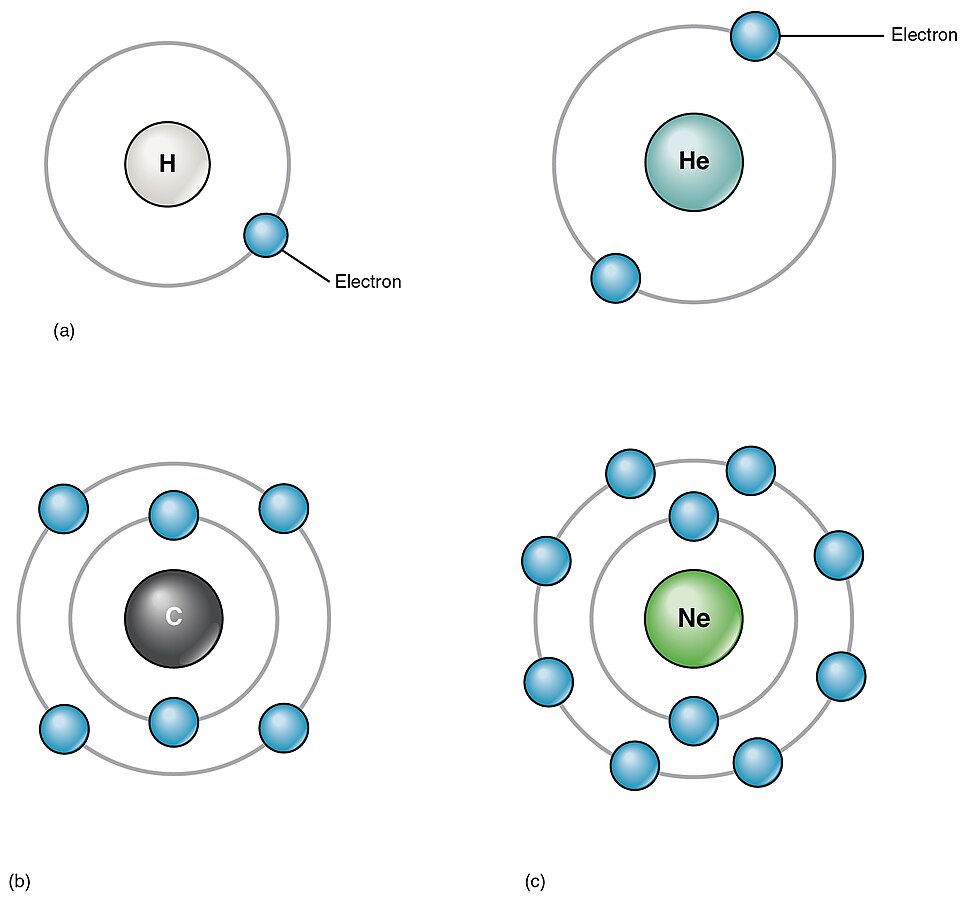
The element atoms found in the human body have from one to five electron shells. The first electron shell holds two electrons. This is always filled with electrons first before any other shell can be filled. Every shell after that holds eight electrons, and the number of shells depends on the number of electrons in the atom.
- Examples:
- Lithium (Li), atomic number 3, has 3 electrons. Two electrons fill the first shell, and the third electron is in the second shell.
- Carbon (C), atomic number 6, has 6 electrons. Two electrons fill the first shell, and four electrons are in the second shell.
- Neon (Ne), atomic number 10, has 10 electrons. Two electrons fill the first shell, and the remaining eight electrons fill the second shell.
See Figure 2.6[9] for an illustration of electron shells.
How elements interact with one another depends on how their electrons are arranged and how many electrons exist in its outermost electron shell. A valence shell is an atom’s outermost electron shell. If the valence shell is full, the atom is stable, and its electrons are unlikely to be pulled away from the nucleus by the electrical charge of other atoms. If the valence shell is not full, the atom is reactive. It will try to react with other atoms to make the valence shell full.
- For example: Hydrogen (atomic number 1) has one electron, which only half-fills its valence shell. This single electron is likely to join with atoms of other elements, so that hydrogen’s single valence shell can be full and thus stabilized.
All atoms (except hydrogen and helium with their single electron shells) are most stable when there are exactly eight electrons in their valence shell. This principle is referred to as the octet rule. The octet rule states that an atom will give up, gain, or share electrons with another atom so that it ends up with eight electrons in its own valence shell.
- For example: Oxygen, with two electrons in the first shell and six electrons in its valence shell, is likely to react with other atoms to add two electrons to oxygen’s valence shell, bringing the number to eight.
In nature, atoms of one element tend to join with atoms of other elements in certain ways.
- Examples:
- Carbon commonly fills its valence shell by linking up with four atoms of hydrogen, forming the simplest of organic molecules, methane, which also is one of the most abundant and stable carbon-containing compounds on Earth.
- Water is formed because oxygen needs two electrons to fill its valence shell. It commonly interacts with two atoms of hydrogen, forming H2O.
Atoms must come close enough for the electrons in their valence shells to interact. However, most physicists would say that they never touch each other because the negatively charged electrons in their valence shells repel one another. Instead, atoms link by forming a chemical bond.
- Betts, J. G., Desaix, P., Johnson, E., Johnson, J. E., Korol, O., Kruse, D., Poe, B., Wise, J., Womble, M. D., & Young, K. A. (2022). Anatomy and physiology, 2e. OpenStax. https://openstax.org/books/anatomy-and-physiology-2e/pages/1-introduction ↵
- “201_Elements_of_the_Human_Body-01” by OpenStax College is licensed under CC BY 3.0 ↵
- “OSC_Microbio_00_AA_atom” by CNX OpenStax is licensed under CC BY 4.0 ↵
- “202_Two_Models_of_Atomic_Structure” by OpenStax College is licensed under CC BY 3.0 ↵
- “CNX_UPhysics_00_CC_Periodic_img” by OpenStax University Physics is licensed under CC BY 4.0 ↵
- Fowler, S., Roush, R., & Wise, J. (2013). Concepts of biology. OpenStax. https://openstax.org/books/concepts-biology/pages/1-introduction ↵
- “204_Isotopes_of_Hydrogen-01” by OpenStax College is licensed under CC BY 3.0 ↵
- Fowler, S., Roush, R., & Wise, J. (2013). Concepts of biology. OpenStax. https://openstax.org/books/concepts-biology/pages/1-introduction ↵
- “206_Electron_Shells-01” by OpenStax College is licensed under CC BY 3.0 ↵
Anything that takes up space and has mass.
A pure substance that cannot be created or broken down by ordinary chemical means or transformed chemically into different substances.
The smallest amount of an element.
A substance composed of two or more elements joined by chemical bonds.
The smallest unit of matter that has the properties of an element.
Positively-charged particles found in the nucleus or center of the atom.
Found in the center or nucleus of an atom, do not have a charge so are neutral and they have a mass of 1.
Very small, negatively-charged particles found outside of the atom’s nucleus. They have no mass and a charge of -1.
The number of protons in the nucleus of an atom of that element. It uniquely identifies the element.
The total number of protons and neutrons in the element’s nucleus.
A chart that organizes all known chemical elements based on their atomic number, electron configuration, and recurring chemical properties.
Different forms of an element, distinguished from one another by different numbers of neutrons.
Also known as a radioisotope, is an atom with an unstable nucleus that undergoes radioactive decay, emitting radiation in the form of alpha particles, beta particles, or gamma rays. This process occurs as the atom seeks a more stable energy state.
A layer of electrons that circle the nucleus at a distinct energy level.
An atom’s outermost electron shell.
A rule that states that an atom will give up, gain, or share electrons with another atom so that it ends up with eight electrons in its own valence shell.

